Abstract
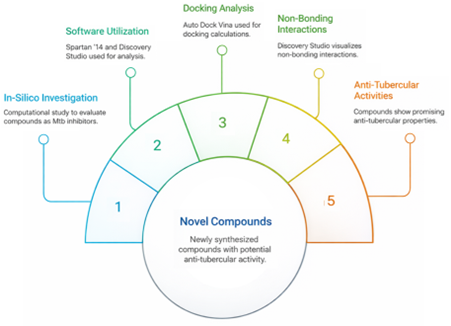
3-(Benzo[d]thiazol-2-yl)-2-((substituted aryl) thiazolidin-4-one derivatives were recently synthesized with thioglycolic acid and evaluated for in vitro anti-tubercular activity. Two of the derivatives have good anti-tubercular activity. The present study evaluated an in-silico investigation of these ten novel compounds as potential Mycobacterium tuberculosis H37Rv inhibitors. The non-bonding interactions between the derivatives and the receptor were studied. Spartan ‘14 software was used for optimization. Discovery Studio software was used for the receptor treatment. The binding site in the downloaded protein was located using Autodock Tool software. Auto Dock Vina was used to calculate the docking, and Discovery Studio was used to view the non-bonding interactions between the docked complexes. Different other parameters were calculated to describe anti-tubercular activities of 3-(benzo[d]thiazol-2-yl)-2-(substituted aryl)thiazolidin-4-one derivatives. The findings demonstrated the potential anti-tubercular properties of all the substances under study and inhibited Mycobacterium tuberculosis (H37Rv). The calculated binding affinity of the docked compound showed improved inhibition against Mycobacterium tuberculosis (H37Rv) better than the standard drugs (Streptomycin and Pyrazinamide), with compound 6 being the best.
References
Abdul-Hammed, M.; Semire, B.; Adegboyega, S. A.; Oyebamiji, A. K.; Olowolafe, T. A. Inhibition of cyclooxygenase-2 and thymidylate synthase by dietary sphingomyelins: insights from DFT and Molecular docking studies. Phys. Chem. Res. 2020, 8 (2), 296–310. https://doi.org/10.22036/pcr.2020.214026.1717
Abdullahi, B. U.; Adamu, U.; Sani, U.; Gideon, A. S. QSAR and Docking Studies on Some Potential Anti-Cancer Agents to Predict their Efect on M14 Melanoma Cell Line. Chem. Afri. 2020, 3, 1009–1022. https://doi.org/10.1007/s42250-020-00185-w
Aziz, M. N.; Patel, A.; Iskander, A.; Chini, A.; Gout, D.; Mandal, S. S.; Lovely, C. J. One-Pot Synthesis of Novel 2-Imino-5-Arylidine-Thiazolidine Analogues and Evaluation of Their Anti-Proliferative Activity against MCF7 Breast Cancer Cell Line. Molecules. 2022a, 27 (3), 841. https://doi.org/10.3390/molecules27030841
Aziz, N. A. A. M.; George, R. F.; El-Adl, K.; Mahmoud, W. R. Design, synthesis, in silico docking, ADMET and anticancer evaluations of thiazolidine-2,4-diones bearing heterocyclic rings as dual VEGFR-2/EGFRT790M tyrosine kinase inhibitors. RSC Adv. 2022b, 12 (20), 12913–12931. https://doi.org/10.1039/D2RA01119K
Bahrami, K.; Khodaei, M.; Naali, F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J. Org. Chem. 2008, 73 (17), 6835–6837. https://doi.org/10.1021/jo8010232
Behr, M. A.; Edelstein, P. H.; Ramakrishnan, L. Is Mycobacterium tuberculosis infection life long? BMJ. 2019, 367, l5770. https://doi.org/10.1136/bmj.l5770
Bhoge, N.; Magare, B.; Mohite, P. Synthesis and Biological Evaluation of 3 (Benzo[d]Thiazol-2-yl)-2-(Substituted Aryl) Thiazolidin-4-one Derivatives. Letters in Applied NanoBioScience. 2024, 13 (1), 19. https://doi.org/10.33263/LIANBS131.019
Chandra, P.; Grigsby S. J.; Philips, J. A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. https://doi.org/10.1038/s41579-022-00763-4
Dernovšek, J.; Zajec, Ž.; Durcik, M.; Mašič, L. P.; Gobec, M.; Zidar, N.; Tomašič, T. Structure-Activity Relationships of Benzothiazole-Based Hsp90 C-Terminal-Domain Inhibitors. Pharmaceutics. 2021, 13 (8), 1283. https://doi.org/10.3390/pharmaceutics13081283
Dookie, N.; Ngema, S. L.; Perumal, R.; Naicker, N.; Padayatchi, N.; Naidoo, K. The changing paradigm of drug-resistant tuberculosis treatment: successes, pitfalls, and future perspectives. Clin. Microbiol. Rev. 2022, 35 (4), e0018019. https://doi.org/10.1128/cmr.00180-19
Fadare, R. I.; Akpor, O. A.; Ifechukwude, I. G.; Richard, D. A.; Bello, C. B. Nurses’ Safety in Caring for Tuberculosis Patients at a Teaching Hospital in South-West Nigeria. Environ. Public Health. 2020, 2020, 3402527. https://doi.org/10.1155/2020/3402527
Ferrer, N. L.; Gómez, A. B.; Soto, C. Y.; Neyrolles, O.; Gicquel, B.; García-Del, Portillo F, Martín C. Intracellular replication of attenuated Mycobacterium tuberculosis phoP mutant in the absence of host cell cytotoxicity. Microbes Infect. 2009, 11 (11), 115–122. https://doi.org/10.1016/j.micinf.2008.10.013
Froes, T. Q.; Chaves, B. T.; Mendes, M. S.; Ximenes, R. M.; da Silva, I. M.; da Silva, P. B. G.; de Albuquerque, J. F. C.; Castilho, M. S. Synthesis and biological evaluation of thiazolidinedione derivatives with high ligand efficiency to P. aeruginosa PhzS. J. Enzyme Inhib. Med. Chem. 2021, 36 (1), 1217–1229. https://doi.org/10.1080/14756366.2021.1931165
Haider, K.; Rehman, S.; Pathak, A.; Najmi, A. K.; Yar, M. S. Advances in 2-substituted benzothiazole scaffold-based chemotherapeutic agents. Arch Pharm. 2021, 354 (12), e2100246. https://doi.org/10.1002/ardp.202100246
Haider, K.; Shrivastava, N.; Pathak, A.; Prasad, Dewa R.; Yahya, S.; Shahar Yar, M. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Results Chem. 2022, 4, 100258. https://doi.org/10.1016/j.rechem.2021.100258
Kumar, S.; Rathore, D.; Garg, G.; Khatri, K.; Saxena, R.; Sahu, S. K. Synthesis and Evaluation of Some Benzothiazole Derivatives as Antidiabetic Agents. Int. J. Pharm. Pharm. Sci. 2017, 9 (2), 60–68. https://doi.org/10.22159/ijpps.2017v9i2.14359
Lawson, H. D.; Walton, S. P.; Chan, C. Metal–organic frameworks for drug delivery: a design perspective. ACS Appl. Mater. Interfaces. 2021, 13 (6), 7004–7020. https://doi.org/10.1021/acsami.1c01089
Lerner, T. R.; Queval, C. J.; Lai, R. P.; Russell, M. R. G.; Fearns, A.; Greenwood, D. J.; Collinson, L.; Wilkinson, R. J.; Gutierrez, M. G. Mycobacterium tuberculosis cords within lymphatic endothelial cells to evade host immunity. JCI Insight. 2020, 5 (10), e136937. https://doi.org/10.1172/jci.insight.136937
Levshin, I. B.; Simonov, A. Y.; Lavrenov, S. N.; Panov, A. A.; Grammatikova, N. E.; Alexandrov, A. A.; Ghazy, E. S. M. O.; Savin, N. A.; Gorelkin, P. V.; Erofeev, A. S.; Polshakov, V. I. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals (Basel). 2022, 15 (5), 563. https://doi.org/10.3390/ph15050563
Lewis, D. F. V.; Broughton, H. B. Molecular binding interactions: their estimation and rationalization in QSARs in terms of theoretically derived parameters. Sci. World J. 2002, 2 (1), 1654–1660. https://doi.org/10.1100/tsw.2002.343
Lončarić, M.; Strelec, I.; Pavić, V.; Rastija, V.; Karnaš, M.; Molnar, M. Green Synthesis of Thiazolidine-2,4-dione Derivatives and Their Lipoxygenase Inhibition Activity with QSAR and Molecular Docking Studies. Front. Chem. 2022, 10, 912822. https://doi.org/10.3389/fchem.2022.912822
Menzies, N. A.; Wolf, E.; Connors, D.; Bellerose, M.; Sbarra, A. N.; Cohen, T.; Hill, A. N.; Yaesoubi, R.; Galer, K.; White, P. J.; Abubakar, I.; Salomon, J. A. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect Dis. 2018, 18 (8), e228–e238. https://doi.org/10.1016/S1473-3099(18)30134-8
Moodley, R.; Mashaba, C.; Rakodi, GH.; Ncube, N. B.; Maphoru, M. V.; Balogun, M. O.; Jordan, A.; Warner, D. F.; Khan, R.; Tukulula, M. New Quinoline-Urea-Benzothiazole Hybrids as Promising Antitubercular Agents: Synthesis, In Vitro Antitubercular Activity, Cytotoxicity Studies, and In Silico ADME Profiling. Pharmaceuticals. 2022, 5 (5), 576. https://doi.org/10.3390/ph15050576
Moule, M. G.; Cirillo, J. D. Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 65. https://doi.org/10.3389/fcimb.2020.00065
Nasr, A. Z.; Farahat, A.; Zein, M. A.; Abdelrehim, E. Synthesis and Antimicrobial Activity of 1,3,4-Oxadiazoline, 1,3-Thiazolidine, and 1,2,4-Triazoline Double-Tailed Acyclo. C-Nucleosides. ACS Omega. 2022, 7 (20), 16884–16894. https://doi.org/10.1021/acsomega.1c06339
Oladipo, S. D.; Tolufashe, G. F.; Mocktar, C.; Omondi, B. Ag(I) symmetrical N, N′-diarylformamidine dithiocarbamate PPh3 complexes: Synthesis, structural characterization, quantum chemical calculations and in vitro biological studies. Inorganica Chim. Acta. 2021, 520, 120316. https://doi.org/10.1016/j.ica.2021.120316
Olasupo, S. B.; Uzairu, A.; Shallangwa, G. A.; Uba, S. Computer-aided drug design and in silico pharmacokinetics predictions of some potential antipsychotic agents. Sci. Afri. 2021, 12, e00734. https://doi.org/10.1016/j.sciaf.2021.e00734
Olujinmi, F. E.; Aworinde, J. O.; Oke, D. G.; Olalekan, O.; Oyebamiji, A. K. Biochemical Evaluation of Potential Antibacterial Activities of (2, 6-Diethylphenyl)-5-Oxopyrrolidine Derivatives via In-Silico Study. Journal of Hunan University Natural Sciences. 2024, 51 (7), 169–177. https://doi.org/10.55463/issn.1674-2974.51.7.16
Oyebamiji, K. A.; Semire, B. Studies of 1, 4-Dihydropyridine Derivatives for Anti-Breast Cancer (MCF-7) Activities: Combinations of DFT-QSAR and Docking Methods. New York Science Journal. 2016, 9 (6), 58-66. https://doi.org/10.7537/marsnys09061610
Oyebamiji, A. K.; Banjo, S. In Vitro Biological Estimation of 1,2,3-Triazolo[4,5-d]pyrimidine Derivatives as Anti-breast Cancer Agent: DFT, QSAR and Docking Studies. Curr. Pharm. Biotechnol. 2020, 21 (1), 70–78. https://doi.org/10.2174/1389201020666190904163003
Oyebamiji, A. K.; Akintelu, A. S.; Mutiu, O. A.; Adeosun, I. J.; Kaka, M. O.; Olotu, T. M.; Soetan, A. E.; Adelowo, J. M.; Semire, B. In-Silico Study on Anti-cancer Activity of Selected Alkaloids from Catharanthus roseus. Trop. J. Nat. Prod. Res. 2021, 5 (7), 1315–1322. https://doi.org/10.26538/tjnpr/v5i7.25
Oyebamiji, A. K.; Olujinmi, F. E.; Aworinde, H. O.; Oke, D. G.; Akintelu, S. A.; Akintayo, E. T.; Akintayo, C. O.; Babalola, J. O. Dataset on anti-human insulin-degrading enzyme activities of cyclic tetra peptides: Insight from in silico approach. Data in Brief. 2024, 55, 110724. https://doi.org/10.1016/j.dib.2024.110724.
Oyebamiji, A.K.; Akintelu; S.A.; Bello-Ogunesan, K.O.; Afolabi, S.O.; Adegoke, K.A; Ebenezer, O.; Akintayo, C.O.; Akintayo, E.T. Mucuna pruriens (L.)- A Potential Phospholipase A2 Inhibitor: In silico Approach. Letter in Applied Nanobioscience, 2025, 14 (3), 195. https://doi.org/10.33263/LIANBS143.195
Oyewole, R. O.; Oyebamiji, A. K.; Semire, B. Theoretical calculations of molecular descriptors for anticancer activities of 1, 2, 3-triazole-pyrimidine derivatives against gastric cancer cell line (MGC-803): DFT, QSAR and docking approaches. Heliyon. 2020, 6 (5), e03926. https://doi.org/10.1016/j.heliyon.2020.e03926
Ren, Y.-S.; Li, H.-L.; Piao, X.-H.; Yang, Z.-Y.; Wang, S.-M.; Ge, Y.-W. Drug affinity responsive target stability (DARTS) accelerated small molecules target discovery: Principles and application. Biochem. Pharmacol. 2021, 194, 114798. https://doi.org/10.1016/j.bcp.2021.114798
Salina, E. G.; Postiglione, U.; Chiarelli, L. R.; Recchia, D.; Záhorszká, M.; Lepioshkin, A.; Monakhova, N.; Pál, A.; Porta, A.; Zanoni, G.; Korduláková, J.; Kazakova, E.; Sassera, D.; Pasca, M. R.; Makarov, V.; Degiacomi, G. A New Benzothiazolthiazolidine Derivative, 11726172, Is Active In Vitro, In Vivo, and against Nonreplicating Cells of Mycobacterium tuberculosis. mSphere. 2022, 7 (6), e00369-22. https://doi.org/10.1128/msphere.00369-22
Setiabudiawan, T. P.; Reurink, R. K.; Hill, P. C.; Netea, M. G.; van Crevel, R.; Koeken, V. A. Protection against tuberculosis by Bacillus Calmette-Guérin (BCG) vaccination: A historical perspective. Med. 2022, 3 (1), 6–24. https://doi.org/10.1016/j.medj.2021.11.006
Shainyan, B. A.; Zhilitskaya, L. V.; Yarosh, N. O. Synthetic Approaches to Biologically Active C-2-Substituted Benzothiazoles. Molecules. 2022, 27 (8), 2598. https://doi.org/10.3390/molecules27082598
Shen, J.; Cheng, F.; Xu, Y.; Li, W.; Tang, Y. Estimation of ADME properties with substructure pattern recognition. J Chem Inf Model. 2010, 50 (6), 1034–1041. https://doi.org/10.1021/ci100104j
Stremski, Y.; Statkova-Abeghe, S.; Kirkova, D.; Angelov, P.; Ivanov, I. Synthesis and structure of new benzothiazole hybrids as potential anticancer agents. Journal of International Scientific Publications. 2021, 15, 173–187.
Sucheta, T. S.; Verma, P. K. Biological potential of thiazolidinedione derivatives of synthetic origin. Chemistry Central Journal. 2017, 11, 130. https://doi.org/10.1186/s13065-017-0357-2
Swalehe, H. M.; Obeagu, E. I. Tuberculosis: Current Diagnosis and Management. Elite Journal of Public Health. 2024, 2 (1), 23–33.
Taghour, M. S.; Elkady, H.; Eldehna, W. M.; El-Deeb, N. M.; Kenawy, A. M.; Elkaeed, E. B.; Alsfouk, A. A.; Alesawy, M. S.; Metwaly, A. M.; Eissa, I. H. Design and synthesis of thiazolidine-2,4-diones hybrids with 1,2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: in-vitro anticancer evaluation and in-silico studies. J. Enzyme. Inhib. Med. Chem. 2022, 37, 1903–1917. https://doi.org/10.1080/14756366.2022.2085693
Tian, Q.; Quan, P.; Fang, L.; Xu, H.; Liu, C. A molecular mechanism investigation of the transdermal/topical absorption classification system on the basis of drug skin permeation and skin retention. Int. J. Pharm. 2021, 608, 121082. https://doi.org/10.1016/j.ijpharm.2021.121082
Trajman, A.; Felker, I.; Alves, L. C.; Coutinho, I.; Osman, M.; Meehan, S. A.; Singh, U. B.; Schwartz, Y. The COVID-19 and TB syndemic: the way forward. Int. J. Tuberc. Lung Dis. 2022, 26 (8), 710–719. https://doi.org/10.5588/ijtld.22.0006
Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Design, synthesis and antimycobacterial activity of thiazolidine-2,4-dione-based thiosemicarbazone derivatives. Bioorganic Chemistry. 2020, 97, 103676. https://doi.org/10.1016/j.bioorg.2020.103676
Trott, O.; Olson, A. J. Autodock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010, 31 (2), 455–461. https://doi.org/10.1002/jcc.21334
Umeokonkwo, C. D.; Okedo-Alex, I. N.; Azuogu, B. N.; Utulu, R, Adeke AS, Disu YO. Trend and determinants of tuberculosis treatment outcome in a tertiary hospital in Southeast Nigeria. J. Infect. Public. Health. 2020, 13 (7), 1029–1033. https://doi.org/10.1016/j.jiph.2019.10.012
World Health Organization (WHO). Strategic and Technical Advisory Group for Tuberculosis (STAG-TB): report of the 21st meeting, 21–23, 2021. Geneva, Switzerland. World Health Organization. https://apps.who.int/iris/handle/10665/351132 (accessed 2021-12-31).
World Health Organization (WHO). Global Tuberculosis Report 2021. Geneva, Switzerland. World Health Organization. https://www.who.int/publications/i/item/9789240037021 (accessed 2022-09-22).
World Health Organization (WHO). WHO Global Task Force on TB Impact Measurement: report of a subgroup meeting on methods used by WHO to estimate TB disease burden, 11-12 May 2022, Geneva, Switzerland. World Health Organization. https://iris.who.int/items/27b1c68a-efd3-48d8-8343-c6d0cb90afd8 (accessed 2022-10-10).
Zhilitskaya, L. V.; Shainyan, B. A.; Yarosh, N. O. Modern Approaches to the Synthesis and Transformations of Practically Valuable Benzothiazole Derivatives. Molecules. 2021, 26 (8), 2190. https://doi.org/10.3390/molecules26082190
Zhilitskaya, L. V.; Yarosh, N. О. Synthesis of biologically active derivatives of 2-aminobenzothiazole. Chem. Heterocycl. Compd. 2021, 57, 369–373. https://doi.org/10.1007/s10593-021-02914-6

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2025 Eclética Química