Resumo
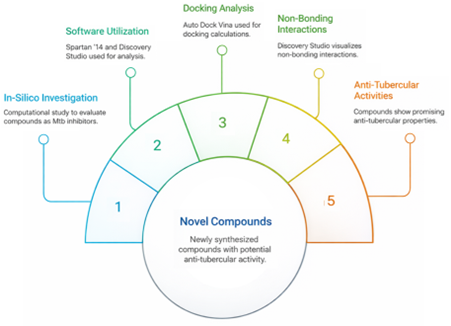
Derivados de 3-(benzo[d]tiazol-2-il)-2-((aril substituído) tiazolidin-4-ona foram recentemente sintetizados com ácido tioglicólico e avaliados quanto à atividade antituberculosa in vitro. Dois dos derivados apresentam boa atividade antituberculosa. O presente estudo avaliou uma investigação in silico desses dez novos compostos como potenciais inibidores do Mycobacterium tuberculosis H37Rv. Foram estudadas as interações não covalentes entre os derivados e o receptor. O software Spartan '14 foi utilizado para otimização. O software Discovery Studio foi utilizado para o tratamento do receptor. O local de ligação na proteína baixada foi localizado utilizando o software Autodock Tool. O Auto Dock Vina foi usado para calcular a ligação, e o Discovery Studio foi usado para visualizar as interações não covalentes entre os complexos ligados. Diferentes outros parâmetros foram calculados para descrever as atividades antituberculosas dos derivados 3-(benzo[d]tiazol-2-il)-2-(aril substituído)tiazolidin-4-ona. Os resultados demonstraram as propriedades antituberculosas potenciais de todas as substâncias em estudo e inibiram o Mycobacterium tuberculosis (H37Rv). A afinidade de ligação calculada do composto acoplado mostrou uma inibição melhorada contra o Mycobacterium tuberculosis (H37Rv) em comparação com os medicamentos padrão (estreptomicina e pirazinamida), sendo o composto 6 o melhor.
Referências
Abdul-Hammed, M.; Semire, B.; Adegboyega, S. A.; Oyebamiji, A. K.; Olowolafe, T. A. Inhibition of cyclooxygenase-2 and thymidylate synthase by dietary sphingomyelins: insights from DFT and Molecular docking studies. Phys. Chem. Res. 2020, 8 (2), 296–310. https://doi.org/10.22036/pcr.2020.214026.1717
Abdullahi, B. U.; Adamu, U.; Sani, U.; Gideon, A. S. QSAR and Docking Studies on Some Potential Anti-Cancer Agents to Predict their Efect on M14 Melanoma Cell Line. Chem. Afri. 2020, 3, 1009–1022. https://doi.org/10.1007/s42250-020-00185-w
Aziz, M. N.; Patel, A.; Iskander, A.; Chini, A.; Gout, D.; Mandal, S. S.; Lovely, C. J. One-Pot Synthesis of Novel 2-Imino-5-Arylidine-Thiazolidine Analogues and Evaluation of Their Anti-Proliferative Activity against MCF7 Breast Cancer Cell Line. Molecules. 2022a, 27 (3), 841. https://doi.org/10.3390/molecules27030841
Aziz, N. A. A. M.; George, R. F.; El-Adl, K.; Mahmoud, W. R. Design, synthesis, in silico docking, ADMET and anticancer evaluations of thiazolidine-2,4-diones bearing heterocyclic rings as dual VEGFR-2/EGFRT790M tyrosine kinase inhibitors. RSC Adv. 2022b, 12 (20), 12913–12931. https://doi.org/10.1039/D2RA01119K
Bahrami, K.; Khodaei, M.; Naali, F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J. Org. Chem. 2008, 73 (17), 6835–6837. https://doi.org/10.1021/jo8010232
Behr, M. A.; Edelstein, P. H.; Ramakrishnan, L. Is Mycobacterium tuberculosis infection life long? BMJ. 2019, 367, l5770. https://doi.org/10.1136/bmj.l5770
Bhoge, N.; Magare, B.; Mohite, P. Synthesis and Biological Evaluation of 3 (Benzo[d]Thiazol-2-yl)-2-(Substituted Aryl) Thiazolidin-4-one Derivatives. Letters in Applied NanoBioScience. 2024, 13 (1), 19. https://doi.org/10.33263/LIANBS131.019
Chandra, P.; Grigsby S. J.; Philips, J. A. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. https://doi.org/10.1038/s41579-022-00763-4
Dernovšek, J.; Zajec, Ž.; Durcik, M.; Mašič, L. P.; Gobec, M.; Zidar, N.; Tomašič, T. Structure-Activity Relationships of Benzothiazole-Based Hsp90 C-Terminal-Domain Inhibitors. Pharmaceutics. 2021, 13 (8), 1283. https://doi.org/10.3390/pharmaceutics13081283
Dookie, N.; Ngema, S. L.; Perumal, R.; Naicker, N.; Padayatchi, N.; Naidoo, K. The changing paradigm of drug-resistant tuberculosis treatment: successes, pitfalls, and future perspectives. Clin. Microbiol. Rev. 2022, 35 (4), e0018019. https://doi.org/10.1128/cmr.00180-19
Fadare, R. I.; Akpor, O. A.; Ifechukwude, I. G.; Richard, D. A.; Bello, C. B. Nurses’ Safety in Caring for Tuberculosis Patients at a Teaching Hospital in South-West Nigeria. Environ. Public Health. 2020, 2020, 3402527. https://doi.org/10.1155/2020/3402527
Ferrer, N. L.; Gómez, A. B.; Soto, C. Y.; Neyrolles, O.; Gicquel, B.; García-Del, Portillo F, Martín C. Intracellular replication of attenuated Mycobacterium tuberculosis phoP mutant in the absence of host cell cytotoxicity. Microbes Infect. 2009, 11 (11), 115–122. https://doi.org/10.1016/j.micinf.2008.10.013
Froes, T. Q.; Chaves, B. T.; Mendes, M. S.; Ximenes, R. M.; da Silva, I. M.; da Silva, P. B. G.; de Albuquerque, J. F. C.; Castilho, M. S. Synthesis and biological evaluation of thiazolidinedione derivatives with high ligand efficiency to P. aeruginosa PhzS. J. Enzyme Inhib. Med. Chem. 2021, 36 (1), 1217–1229. https://doi.org/10.1080/14756366.2021.1931165
Haider, K.; Rehman, S.; Pathak, A.; Najmi, A. K.; Yar, M. S. Advances in 2-substituted benzothiazole scaffold-based chemotherapeutic agents. Arch Pharm. 2021, 354 (12), e2100246. https://doi.org/10.1002/ardp.202100246
Haider, K.; Shrivastava, N.; Pathak, A.; Prasad, Dewa R.; Yahya, S.; Shahar Yar, M. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Results Chem. 2022, 4, 100258. https://doi.org/10.1016/j.rechem.2021.100258
Kumar, S.; Rathore, D.; Garg, G.; Khatri, K.; Saxena, R.; Sahu, S. K. Synthesis and Evaluation of Some Benzothiazole Derivatives as Antidiabetic Agents. Int. J. Pharm. Pharm. Sci. 2017, 9 (2), 60–68. https://doi.org/10.22159/ijpps.2017v9i2.14359
Lawson, H. D.; Walton, S. P.; Chan, C. Metal–organic frameworks for drug delivery: a design perspective. ACS Appl. Mater. Interfaces. 2021, 13 (6), 7004–7020. https://doi.org/10.1021/acsami.1c01089
Lerner, T. R.; Queval, C. J.; Lai, R. P.; Russell, M. R. G.; Fearns, A.; Greenwood, D. J.; Collinson, L.; Wilkinson, R. J.; Gutierrez, M. G. Mycobacterium tuberculosis cords within lymphatic endothelial cells to evade host immunity. JCI Insight. 2020, 5 (10), e136937. https://doi.org/10.1172/jci.insight.136937
Levshin, I. B.; Simonov, A. Y.; Lavrenov, S. N.; Panov, A. A.; Grammatikova, N. E.; Alexandrov, A. A.; Ghazy, E. S. M. O.; Savin, N. A.; Gorelkin, P. V.; Erofeev, A. S.; Polshakov, V. I. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals (Basel). 2022, 15 (5), 563. https://doi.org/10.3390/ph15050563
Lewis, D. F. V.; Broughton, H. B. Molecular binding interactions: their estimation and rationalization in QSARs in terms of theoretically derived parameters. Sci. World J. 2002, 2 (1), 1654–1660. https://doi.org/10.1100/tsw.2002.343
Lončarić, M.; Strelec, I.; Pavić, V.; Rastija, V.; Karnaš, M.; Molnar, M. Green Synthesis of Thiazolidine-2,4-dione Derivatives and Their Lipoxygenase Inhibition Activity with QSAR and Molecular Docking Studies. Front. Chem. 2022, 10, 912822. https://doi.org/10.3389/fchem.2022.912822
Menzies, N. A.; Wolf, E.; Connors, D.; Bellerose, M.; Sbarra, A. N.; Cohen, T.; Hill, A. N.; Yaesoubi, R.; Galer, K.; White, P. J.; Abubakar, I.; Salomon, J. A. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect Dis. 2018, 18 (8), e228–e238. https://doi.org/10.1016/S1473-3099(18)30134-8
Moodley, R.; Mashaba, C.; Rakodi, GH.; Ncube, N. B.; Maphoru, M. V.; Balogun, M. O.; Jordan, A.; Warner, D. F.; Khan, R.; Tukulula, M. New Quinoline-Urea-Benzothiazole Hybrids as Promising Antitubercular Agents: Synthesis, In Vitro Antitubercular Activity, Cytotoxicity Studies, and In Silico ADME Profiling. Pharmaceuticals. 2022, 5 (5), 576. https://doi.org/10.3390/ph15050576
Moule, M. G.; Cirillo, J. D. Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 65. https://doi.org/10.3389/fcimb.2020.00065
Nasr, A. Z.; Farahat, A.; Zein, M. A.; Abdelrehim, E. Synthesis and Antimicrobial Activity of 1,3,4-Oxadiazoline, 1,3-Thiazolidine, and 1,2,4-Triazoline Double-Tailed Acyclo. C-Nucleosides. ACS Omega. 2022, 7 (20), 16884–16894. https://doi.org/10.1021/acsomega.1c06339
Oladipo, S. D.; Tolufashe, G. F.; Mocktar, C.; Omondi, B. Ag(I) symmetrical N, N′-diarylformamidine dithiocarbamate PPh3 complexes: Synthesis, structural characterization, quantum chemical calculations and in vitro biological studies. Inorganica Chim. Acta. 2021, 520, 120316. https://doi.org/10.1016/j.ica.2021.120316
Olasupo, S. B.; Uzairu, A.; Shallangwa, G. A.; Uba, S. Computer-aided drug design and in silico pharmacokinetics predictions of some potential antipsychotic agents. Sci. Afri. 2021, 12, e00734. https://doi.org/10.1016/j.sciaf.2021.e00734
Olujinmi, F. E.; Aworinde, J. O.; Oke, D. G.; Olalekan, O.; Oyebamiji, A. K. Biochemical Evaluation of Potential Antibacterial Activities of (2, 6-Diethylphenyl)-5-Oxopyrrolidine Derivatives via In-Silico Study. Journal of Hunan University Natural Sciences. 2024, 51 (7), 169–177. https://doi.org/10.55463/issn.1674-2974.51.7.16
Oyebamiji, K. A.; Semire, B. Studies of 1, 4-Dihydropyridine Derivatives for Anti-Breast Cancer (MCF-7) Activities: Combinations of DFT-QSAR and Docking Methods. New York Science Journal. 2016, 9 (6), 58-66. https://doi.org/10.7537/marsnys09061610
Oyebamiji, A. K.; Banjo, S. In Vitro Biological Estimation of 1,2,3-Triazolo[4,5-d]pyrimidine Derivatives as Anti-breast Cancer Agent: DFT, QSAR and Docking Studies. Curr. Pharm. Biotechnol. 2020, 21 (1), 70–78. https://doi.org/10.2174/1389201020666190904163003
Oyebamiji, A. K.; Akintelu, A. S.; Mutiu, O. A.; Adeosun, I. J.; Kaka, M. O.; Olotu, T. M.; Soetan, A. E.; Adelowo, J. M.; Semire, B. In-Silico Study on Anti-cancer Activity of Selected Alkaloids from Catharanthus roseus. Trop. J. Nat. Prod. Res. 2021, 5 (7), 1315–1322. https://doi.org/10.26538/tjnpr/v5i7.25
Oyebamiji, A. K.; Olujinmi, F. E.; Aworinde, H. O.; Oke, D. G.; Akintelu, S. A.; Akintayo, E. T.; Akintayo, C. O.; Babalola, J. O. Dataset on anti-human insulin-degrading enzyme activities of cyclic tetra peptides: Insight from in silico approach. Data in Brief. 2024, 55, 110724. https://doi.org/10.1016/j.dib.2024.110724.
Oyebamiji, A.K.; Akintelu; S.A.; Bello-Ogunesan, K.O.; Afolabi, S.O.; Adegoke, K.A; Ebenezer, O.; Akintayo, C.O.; Akintayo, E.T. Mucuna pruriens (L.)- A Potential Phospholipase A2 Inhibitor: In silico Approach. Letter in Applied Nanobioscience, 2025, 14 (3), 195. https://doi.org/10.33263/LIANBS143.195
Oyewole, R. O.; Oyebamiji, A. K.; Semire, B. Theoretical calculations of molecular descriptors for anticancer activities of 1, 2, 3-triazole-pyrimidine derivatives against gastric cancer cell line (MGC-803): DFT, QSAR and docking approaches. Heliyon. 2020, 6 (5), e03926. https://doi.org/10.1016/j.heliyon.2020.e03926
Ren, Y.-S.; Li, H.-L.; Piao, X.-H.; Yang, Z.-Y.; Wang, S.-M.; Ge, Y.-W. Drug affinity responsive target stability (DARTS) accelerated small molecules target discovery: Principles and application. Biochem. Pharmacol. 2021, 194, 114798. https://doi.org/10.1016/j.bcp.2021.114798
Salina, E. G.; Postiglione, U.; Chiarelli, L. R.; Recchia, D.; Záhorszká, M.; Lepioshkin, A.; Monakhova, N.; Pál, A.; Porta, A.; Zanoni, G.; Korduláková, J.; Kazakova, E.; Sassera, D.; Pasca, M. R.; Makarov, V.; Degiacomi, G. A New Benzothiazolthiazolidine Derivative, 11726172, Is Active In Vitro, In Vivo, and against Nonreplicating Cells of Mycobacterium tuberculosis. mSphere. 2022, 7 (6), e00369-22. https://doi.org/10.1128/msphere.00369-22
Setiabudiawan, T. P.; Reurink, R. K.; Hill, P. C.; Netea, M. G.; van Crevel, R.; Koeken, V. A. Protection against tuberculosis by Bacillus Calmette-Guérin (BCG) vaccination: A historical perspective. Med. 2022, 3 (1), 6–24. https://doi.org/10.1016/j.medj.2021.11.006
Shainyan, B. A.; Zhilitskaya, L. V.; Yarosh, N. O. Synthetic Approaches to Biologically Active C-2-Substituted Benzothiazoles. Molecules. 2022, 27 (8), 2598. https://doi.org/10.3390/molecules27082598
Shen, J.; Cheng, F.; Xu, Y.; Li, W.; Tang, Y. Estimation of ADME properties with substructure pattern recognition. J Chem Inf Model. 2010, 50 (6), 1034–1041. https://doi.org/10.1021/ci100104j
Stremski, Y.; Statkova-Abeghe, S.; Kirkova, D.; Angelov, P.; Ivanov, I. Synthesis and structure of new benzothiazole hybrids as potential anticancer agents. Journal of International Scientific Publications. 2021, 15, 173–187.
Sucheta, T. S.; Verma, P. K. Biological potential of thiazolidinedione derivatives of synthetic origin. Chemistry Central Journal. 2017, 11, 130. https://doi.org/10.1186/s13065-017-0357-2
Swalehe, H. M.; Obeagu, E. I. Tuberculosis: Current Diagnosis and Management. Elite Journal of Public Health. 2024, 2 (1), 23–33.
Taghour, M. S.; Elkady, H.; Eldehna, W. M.; El-Deeb, N. M.; Kenawy, A. M.; Elkaeed, E. B.; Alsfouk, A. A.; Alesawy, M. S.; Metwaly, A. M.; Eissa, I. H. Design and synthesis of thiazolidine-2,4-diones hybrids with 1,2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: in-vitro anticancer evaluation and in-silico studies. J. Enzyme. Inhib. Med. Chem. 2022, 37, 1903–1917. https://doi.org/10.1080/14756366.2022.2085693
Tian, Q.; Quan, P.; Fang, L.; Xu, H.; Liu, C. A molecular mechanism investigation of the transdermal/topical absorption classification system on the basis of drug skin permeation and skin retention. Int. J. Pharm. 2021, 608, 121082. https://doi.org/10.1016/j.ijpharm.2021.121082
Trajman, A.; Felker, I.; Alves, L. C.; Coutinho, I.; Osman, M.; Meehan, S. A.; Singh, U. B.; Schwartz, Y. The COVID-19 and TB syndemic: the way forward. Int. J. Tuberc. Lung Dis. 2022, 26 (8), 710–719. https://doi.org/10.5588/ijtld.22.0006
Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Design, synthesis and antimycobacterial activity of thiazolidine-2,4-dione-based thiosemicarbazone derivatives. Bioorganic Chemistry. 2020, 97, 103676. https://doi.org/10.1016/j.bioorg.2020.103676
Trott, O.; Olson, A. J. Autodock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem. 2010, 31 (2), 455–461. https://doi.org/10.1002/jcc.21334
Umeokonkwo, C. D.; Okedo-Alex, I. N.; Azuogu, B. N.; Utulu, R, Adeke AS, Disu YO. Trend and determinants of tuberculosis treatment outcome in a tertiary hospital in Southeast Nigeria. J. Infect. Public. Health. 2020, 13 (7), 1029–1033. https://doi.org/10.1016/j.jiph.2019.10.012
World Health Organization (WHO). Strategic and Technical Advisory Group for Tuberculosis (STAG-TB): report of the 21st meeting, 21–23, 2021. Geneva, Switzerland. World Health Organization. https://apps.who.int/iris/handle/10665/351132 (accessed 2021-12-31).
World Health Organization (WHO). Global Tuberculosis Report 2021. Geneva, Switzerland. World Health Organization. https://www.who.int/publications/i/item/9789240037021 (accessed 2022-09-22).
World Health Organization (WHO). WHO Global Task Force on TB Impact Measurement: report of a subgroup meeting on methods used by WHO to estimate TB disease burden, 11-12 May 2022, Geneva, Switzerland. World Health Organization. https://iris.who.int/items/27b1c68a-efd3-48d8-8343-c6d0cb90afd8 (accessed 2022-10-10).
Zhilitskaya, L. V.; Shainyan, B. A.; Yarosh, N. O. Modern Approaches to the Synthesis and Transformations of Practically Valuable Benzothiazole Derivatives. Molecules. 2021, 26 (8), 2190. https://doi.org/10.3390/molecules26082190
Zhilitskaya, L. V.; Yarosh, N. О. Synthesis of biologically active derivatives of 2-aminobenzothiazole. Chem. Heterocycl. Compd. 2021, 57, 369–373. https://doi.org/10.1007/s10593-021-02914-6

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Copyright (c) 2025 Eclética Química