Abstract
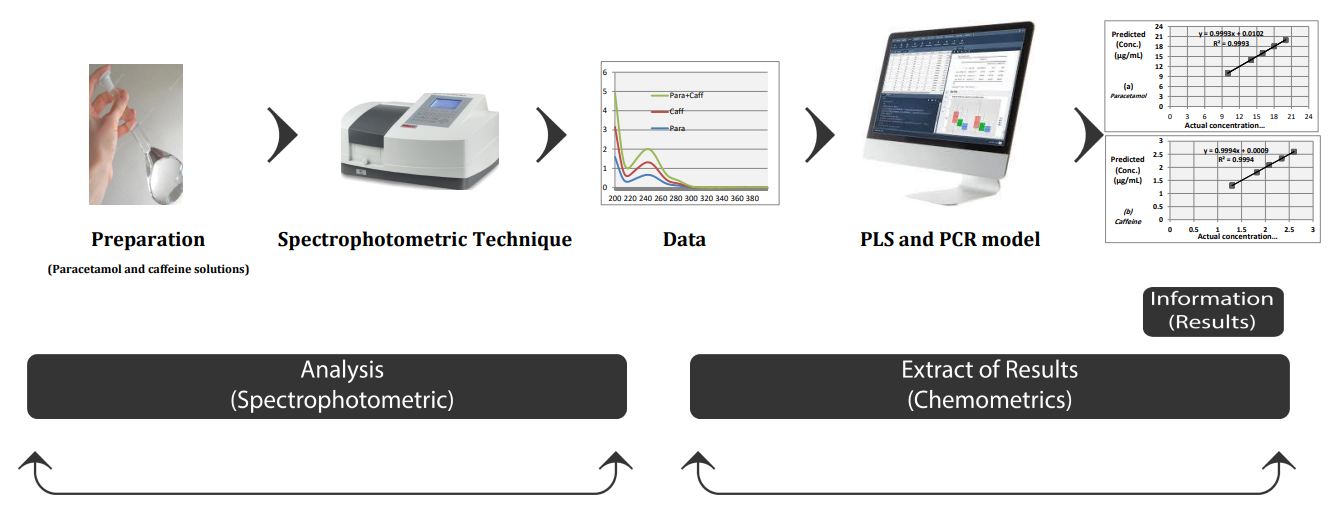
A green analytical method, a simple, fast, and cost-effective simultaneous spectrophotometric method using two chemometric techniques, the partial least square regression (PLS) and principal component regression (PCR), for determining a combination of paracetamol and caffeine in pharmaceutical formulations was developed. Pretreatment and separation steps are not required in the proposed method. For model construction and validation, various drug concentrations and instrumental spectra of 25 mixed solutions of paracetamol and caffeine were analyzed. The UV analysis of the prepared mixtures was recorded for a selected solvent blank in the wavelength range of 210-300 nm. The digitized absorbance was sampled at 0.2-nm intervals. R2 values of 0.9993 and 0.9994 assigned for the PLS of paracetamol and caffeine and 0.9995 and 0.9991 for the PCR of paracetamol and caffeine, respectively, exhibited greater prediction efficiencies. The obtained results were statistically compared with the results of the HPLC reference method. Concerning accuracy and precision, the statistical comparison revealed no significant differences between the suggested and reference HPLC approaches.
References
Aktaş, A. H.; Kitiş, F. Spectrophotometric simultaneous determination of caffeine and paracetamol in commercial pharmaceutical by principal component regression, partial least squares and artificial neural networks chemometric methods. Croat. Chem. Acta. 2014, 87 (1), 69–74. https://doi.org/10.5562/cca2214
Alam, P.; Shakeel, F.; Ali, A.; Alqarni, M. H.; Foudah, A. I.; Aljarba, T. M.; Alkholifi, F. K.; Alshehri, S.; Ghoneim, M. M.; Ali, A. Simultaneous determination of caffeine and paracetamol in commercial formulations using greener normal-phase and reversed-phase HPTLC methods: A contrast of validation parameters. Molecules. 2022, 27 (2), 405. https://doi.org/10.3390/molecules27020405
Aminu, N.; Chan, S.-Y.; Khan, N. H.; Farhan, A. B.; Umar, M. N.; Toh, S.-M. A simple stability-indicating HPLC method for simultaneous analysis of paracetamol and caffeine and its application to determinations in fixed-dose combination tablet dosage form. Acta Chromatogr. 2019, 31 (2), 85–91. https://doi.org/10.1556/1326.2018.00354
Ashour, A.; Hegazy, M. A.; Abdel-Kawy, M.; ElZeiny, M. B. Simultaneous spectrophotometric determination of overlapping spectra of paracetamol and caffeine in laboratory prepared mixtures and pharmaceutical preparations using continuous wavelet and derivative transform. J. Saudi Chem. Soc. 2015, 19 (2), 186–192. https://doi.org/10.1016/j.jscs.2012.02.004
Attia, K. A.-S. M.; Abdel-Aziz, O.; Magdy, N.; Mohamed, G. F. Development and validation of different chemometric-assisted spectrophotometric methods for determination of cefoxitin-sodium in presence of its alkali-induced degradation product. Futur. J. Pharma. Sci. 2018, 4 (2), 241–247. https://doi.org/10.1016/j.fjps.2018.08.002
Belal, F.; Ibrahim, F.; Sheribah, Z.; Alaa, H. New spectrophotometric/chemometric assisted methods for the simultaneous determination of imatinib, gemifloxacin, nalbuphine and naproxen in pharmaceutical formulations and human urine. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2018, 198, 51–60. https://doi.org/10.1016/j.saa.2018.02.048
Darbandi, A.; Sohrabi, M. R.; Bahmaei, M. Development of a chemometric-assisted spectrophotometric method for quantitative simultaneous determination of Amlodipine and Valsartan in commercial tablet. Optik. 2020, 218, 165110. https://doi.org/10.1016/j.ijleo.2020.165110
Drugbank online. Acetaminophen. Drug created at June 13, 2005a 13:24 / Updated at September 07, 2024. https://go.drugbank.com/drugs/DB00316. Accessed on 18 Jan 2023.
Drugbank online. Caffeine. Drug created at June 13, 2005b 13:24 / Updated at September 07, 2024. https://go.drugbank.com/drugs/DB00201. Accessed on 18 Jan 2023.
Elfatatry, H. M.; Mabrouk, M. M.; Hammad, S. F.; Mansour, F. R.; Kamal, A. H.; Alahmad, S. Development and validation of chemometric-assisted spectrophotometric methods for simultaneous determination of phenylephrine hydrochloride and ketorolac tromethamine in binary combinations. J. AOAC Int. 2016, 99 (5), 1247–1251. https://doi.org/10.5740/jaoacint.16-0106
Eticha, T.; Kahsay, G.; Asefa, F.; Hailu, T.; Gebretsadik, H.; Gebretsadikan, T.; Thangabalan, B. Chemometric-Assisted spectrophotometric method for the simultaneous determination of ciprofloxacin and doxycycline hyclate in pharmaceutical formulations. J. Anal. Methods Chem. 2018, 2018, 9538435. https://doi.org/10.1155/2018/9538435
Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC, Trends Anal. Chem. 2013, 50, 78–84. https://doi.org/10.1016/j.trac.2013.04.010
Gandhi, S. V.; Abhilash, D.; Waghmare, N. Y.; Mutha, A. S. Chemometrics-assisted UV spectrophotometric method for determination of ciprofloxacin and ornidazole in pharmaceutical formulation. ARC J. Pharm. Sci. 2017, 3 (1), 19–25. https://doi.org/10.20431/2455-1538.0301005
Gholse, Y. N.; Chaple, D. R.; Kasliwal, R. H. Development and Validation of Novel Analytical Simultaneous Estimation Based UV Spectrophotometric Method for Doxycycline and Levofloxacin Determination. Biointerface Res. Appl. Chem. 2022, 12 (4) 5458–5478. https://doi.org/10.33263/BRIAC124.54585478
Glavanović, S.; Glavanović, M.; Tomišić, V. Simultaneous quantitative determination of paracetamol and tramadol in tablet formulation using UV spectrophotometry and chemometric methods. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2016, 157, 258–264. https://doi.org/10.1016/j.saa.2015.12.020
Manouchehri, F.; Izadmanesh, Y.; Aghaee, E.; Ghasemi, J. B. Experimental, computational and chemometrics studies of BSA-vitamin B6 interaction by UV-Vis, FT-IR, fluorescence spectroscopy, molecular dynamics simulation and hard-soft modeling methods. Bioorg. Chem. 2016, 68, 124–136. https://doi.org/10.1016/j.bioorg.2016.07.014
Mattar, A. A.; Sobhy, M. UV-Chemometric Method Development for Resolving The Overlapped Spectra of Aspirin, Caffeine and Orphenadrine Citrate in Their Ternary Pharmaceutical Dosage Form. Preprint from Research Square, Jan. 31 2022. https://doi.org/10.21203/rs.3.rs-1262160/v1
Mohammed, O. J.; Hamzah, M. J.; Saeed, A. M. RP-HPLC Method Validation for Simultaneous Estimation of Paracetamol and Caffeine in Formulating Pharmaceutical Form. Res. J. Pharm. Technol. 2021, 14 (9), 4743–4748. https://doi.org/10.52711/0974-360X.2021.00825
Moroni, A. B.; Vega, D. R.; Kaufman, T. S.; Calvo, N. L. Form quantitation in desmotropic mixtures of albendazole bulk drug by chemometrics-assisted analysis of vibrational spectra. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2022, 265, 120354. https://doi.org/10.1016/j.saa.2021.120354
Moussa, B. A.; Mahrouse, M. A.; Fawzy, M. G. Smart spectrophotometric methods for the simultaneous determination of newly co-formulated hypoglycemic drugs in binary mixtures. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2021, 257, 119763. https://doi.org/10.1016/j.saa.2021.119763
Muntean, D. M.; Alecu, C.; Tomuta, I. Simultaneous quantification of paracetamol and caffeine in powder blends for tableting by NIR-chemometry. J. Spectrosc. 2017, 2017, 160675. https://doi.org/10.1155/2017/7160675
Muntean, D.; Porfire, A.; Alecu, C.; Iurian, S.; Casian, T.; Gâvan, A.; Tomuta, I. A non-destructive NIR spectroscopic method combined with chemometry for simultaneous assay of paracetamol and caffeine in tablets. Ro. J. Pharm. Pract. 2021, 14 (2), 68–75. https://doi.org/10.37897/RJPhP.2021.2.2
Ortega-Barrales, P.; Padilla-Weigand, R.; Molina-Díaz, A. Simultaneous determination of paracetamol and caffeine by flow injection-solid phase spectrometry using C18 silica gel as a sensing support. Anal. Sci. 2002, 18 (11), 1241-1246. https://doi.org/10.2116/analsci.18.1241
Patel, K. R.; Prajapati, L. M.; Joshi, A. K.; Kharodiya, M. L.; Patel, J. R. Application of Chemometrics in Simultaneous Spectrophotometric Quantification of Etophylline and Theophylline: The Drugs with Same Chromophore. Iran. J. Pharm. Sci. 2013a, 9 (3), 17–28. https://doi.org/10.22037/ijps.v9.40870
Patel, M. N.; Alvi, S. N.; Savalia, M. D.; Kathiria, P. B.; Patel, B. A.; Parmar, S. J. Development and Validation of First Order Derivative Spectrophotometric Method for Simultaneous Estimation of Paracetamol and Caffeine in Tablet Dosage Form. Inventi Rapid: Pharm Analysis & Quality Assurance. 2013b, 5 (5), 213–218.
Phechkrajang, C.; Siriratawan, W.; Narapanich, K.; Thanomchat, K.; Kantanawat, P.; Srikajhondei, W.; Khajornvanitchot, V.; Sakchaisri, K. Development and validation of chemometrics-assisted spectrophotometric method for determination of clotrimazole in the presence of betamethasone valerate. Mahidol Univ. J. Pharm. Sci. 2015, 42, 1–7.
Putri, D. C. A.; Gani, M. R.; Octa, F. D. Chemometrics-Assisted UV Spectrophotometric Method for Simultaneous Determination of Paracetamol and Tramadol in Divided Powder Dosage Form. Inter. J. Pharm. Res. 2021, 13 (01), 1901–1907. https://doi.org/10.31838/ijpr/2021.13.01.075
Rahman, A.; Sravani, G. J.; Srividya, K.; Priyadharshni, A. D. R.; Narmada, A.; Sahithi, K.; Sai, T. K.; Padmavathi, Y. Development and Validation of Chemometric Assisted FTIR Spectroscopic Method for Simultaneous Estimation of Valsartan and Hydrochlorothiazide in Pure and Pharmaceutical Dosage Forms. J. Young Pharm. 2020, 12 (2s), s51–s55. https://doi.org/10.5530/jyp.2020.12s.46
Riddhi, P.; Rajashree, M., Development and Validation of Chemometric Assisted Methods and Stability Indicating RP-HPLC Method for Simultaneous Estimation of Rasagiline Mesylate and Pramipexole in Synthetic Mixture. Acta Sci. Pharm. Sci. 2019, 3 (8), 154–168. https://doi.org/10.31080/ASPS.2019.03.0359
Salem, Y. A.; Hammouda, M. E.; El-Enin, M. A. A.; El-Ashry, S. M. Application of derivative emission fluorescence spectroscopy for determination of ibuprofen and phenylephrine simultaneously in tablets and biological fluids. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2019, 210, 387–397. https://doi.org/10.1016/j.saa.2018.11.054
Sebaiy, M. M.; Sobhy, M.; Mattar, A. A. Different techniques for overlapped UV spectra resolution of some co-administered drugs with paracetamol in their combined pharmaceutical dosage forms. Spectrochim. Acta A. Mol. Biomol. Spectrosc.. 2020, 224, 117429. https://doi.org/10.1016/j.saa.2019.117429
Shah, U. H.; Jasani, A. H. Chemometric assisted spectrophotometric methods for simultaneous determination of paracetamol and tolperisone hydrochloride in pharmaceutical dosage form. Eurasian J. Anal. Chem. 2017, 12 (3), 211–222. https://doi.org/10.12973/ejac.2017.00164a
Shinde, M. A.; Divya, O. Simultaneous quantitative analysis of a three-drug combination using synchronous fluorescence spectroscopy and chemometrics. Curr. Sci. 2015, 1348–1354.
Silva, W. C.; Pereira, P. F.; Marra, M. C.; Gimenes, D. T.; Cunha, R. R.; Da Silva, R. A.; Munoz, R. A.; Richter, E. M. A simple strategy for simultaneous determination of paracetamol and caffeine using flow injection analysis with multiple pulse amperometric detection. Electroanalysis. 2011, 23 (12), 2764-2770. https://doi.org/10.1002/elan.201100512
Singh, V. D.; Singh, V. K. Chemo-metric assisted UV-spectrophotometric methods for simultaneous estimation of Darunavir ethanolate and Cobicistat in binary mixture and their tablet formulation. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2021, 250, 119383. https://doi.org/10.1016/j.saa.2020.119383
Sun, X.; Li, H.; Yi, Y.; Hua, H.; Guan, Y.; Chen, C. Rapid detection and quantification of adulteration in Chinese hawthorn fruits powder by near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2021, 250, 119346. https://doi.org/10.1016/j.saa.2020.119346
Tobiszewski, M.; Namieśnik, J.; Pena-Pereira, F., A derivatisation agent selection guide. Green Chem. 2017, 19 (24), 5911–5922. https://doi.org/10.1039/C7GC03108D
Tsvetkova, B.; Kostova, B.; Pencheva, I.; Zlatkov, A.; Rachev, D.; Peikov, P. Validated LC method for simultaneous analysis of paracetamol and caffeine in model tablet formulation. Int. J. Pharm. Pharm. Sci. 2012, 4 (4), 680–684.
Uddin, M.; Mondal, A.; Karim, M.; Jahan, R.; Rana, A. Chemometrics assisted spectrophotometric method for simultaneous determination of paracetamol and caffeine in pharmaceutical formulations. Bangladesh J. Sci. Ind. Res. 2019, 54 (3), 215–222. https://doi.org/10.3329/bjsir.v54i3.42673
United States Pharmacopeia and the National Formulary (USP 43 - NF 38). The United States Pharmacopeial Convention; 2020.
Vichare, V.; Mujgond, P.; Tambe, V.; Dhole, S. Simultaneous spectrophotometric determination of paracetamol and caffeine in tablet formulation. Int. J. Pharmtech Res. 2010, 2 (4), 2512–2516.
Vu Dang, H.; Truong Thi Thu, H.; Dong Thi Ha, L.; Nguyen Mai, H. RP-HPLC and UV Spectrophotometric Analysis of Paracetamol, Ibuprofen, and Caffeine in Solid Pharmaceutical Dosage Forms by Derivative, Fourier, and Wavelet Transforms: A Comparison Study. J. Anal. Methods Chem. 2020, 2020, 8107571. https://doi.org/10.1155/2020/8107571
Walash, M. I.; Belal, F. F.; El-Enany, N. M.; El-Maghrabey, M. H. Synchronous fluorescence spectrofluorimetric method for the simultaneous determination of metoprolol and felodipine in combined pharmaceutical preparation. Chem. Cent. J. 2011, 5 (1), 70. https://doi.org/10.1186/1752-153X-5-70
Yehia, A. M.; Mohamed, H. M. Chemometrics resolution and quantification power evaluation: application on a pharmaceutical quaternary mixture of Paracetamol, Guaifenesin, Phenylephrine and p-aminophenol. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2016, 152, 491–500. https://doi.org/10.1016/j.saa.2015.07.101
Zhu, L.; Wu, H.-L.; Xie, L.-X.; Fang, H Xiang, S.-X.; Hu, Y.; Liu, Z.; Wanga, T.; Yu, R.-Q. A chemometrics-assisted excitation-emission matrix fluorescence method for simultaneous determination of arbutin and hydroquinone in cosmetic products. Anal. Methods. 2016, 8 (24), 4941–4948. https://doi.org/10.1039/C6AY00821F

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Eclética Química