Abstract
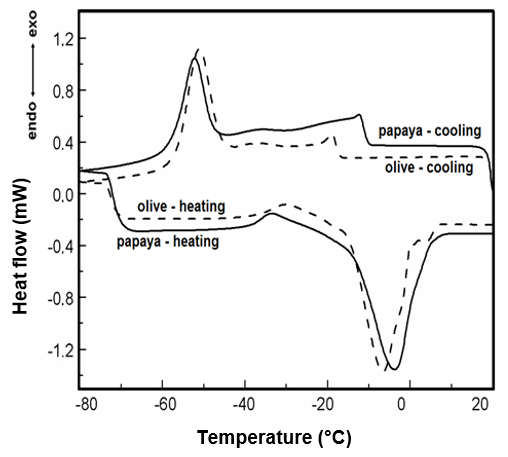
Carica papaya L. is frequently found in northern and northeastern Brazil, the Caribbean and Central America. The papaya seed oil was extracted using Soxhlet extraction. Its oil has low acidity, iodine index, saponification and peroxide values adequate and comparable to other commercially available vegetable oils. The lipid profile of this oil was also obtained by gas chromatography; thermal characterization was performed by thermogravimetry (TG) and differential scanning calorimetry (DSC) under various heating rates. The composition of the in-nature oil indicates that oleic and palmitic fatty acids are predominant. The DSC analysis under cooling shows that the crystallization phase of this oil is similar to olive oil, and the thermogravimetric results show the thermal decomposition under nitrogen purge gas occurs in only one stage. The activation energy was obtained applying the isoconversional methods proposed by Capela and Ribeiro, Ozawa and Friedman. The obtained kinetics data leads to a dependence on the sample mass and purge gases, which results in several kinetic patterns.
References
Anuar, N. S.; Zahari, S. S.; Taib, I. A.; Rahman, M. T. Effect of green and ripe Carica papaya epicarp extracts on wound healing and during pregnancy. Food Chem. Toxic. 2008, 46 (7), 2384–2389. https://doi.org/10.1016/j.fct.2008.03.025
Alobo, A. P. Proximate composition and selected functional properties of defatted papaya (Carica papaya L.) kernel flour. Plant Foods Hum. Nutr. 2003, 58 (3), 1–7. https://doi.org/10.1023/B:QUAL.0000040319.61845.c2
American Society for Testing and Materials (ASTM). D1980–87. Standard Test Method for Acid Value of Fatty Acids and Polymerizes Fatty Acids. ASTM, 1998.
American Society for Testing and Materials (ASTM). D5554–95. Standard Test Method for Determination of the Iodine Value of Fats and Oils. ASTM, 2011a.
American Society for Testing and Materials (ASTM). D5558–95 Standard Test Method for Determination of the Saponification Value of Fats and Oils. ASTM, 2011b.
Damodaran, S.; Parkin, K. L.; Fennema, O. R. Química de Alimentos de Fennema; Artmed, 2010.
Dias, D. S.; Crespi, M. S.; Ribeiro, C. A.; Kobelnik, M. Evaluation by thermogravimetry of the interaction of the poly(ethylene terephthalate) with oil-based paint. Eclét. Quím. 2015, 40 (1), 77–85. https://doi.org/10.26850/1678-4618eqj.v40.1.2015.p77-85
Dias, D. S.; Crespi, M. S.; Ribeiro, C. A.; Kobelnik, M. Evaluation of the thermal decomposition of blends prepared with poly(3‑hydroxybutyrate) (PHB) and recyclable ethylene poly‑terephthalate (RPET). J. Therm. Anal. Calorim. 2021, 143, 3447–3457. https://doi.org/10.1007/s10973-020-09885-4
Fonseca, M; Ferreira, L. M. B.; Soares, R. A. M.; Kobelnik, M.; Fontanari, G. G.; Crespi, M. S.; Ribeiro, C. A. Extraction of soursop oil (Annona muricata L.) by ultrasonic technique. J. Therm. Anal. Calorim. 2018, 134 (3), 1893–1901. https://doi.org/10.1007/s10973-018-7753-2
Fontanari, G. G.; Kobelnik, M.; Marques, M. R.; Arêas, J. A. G.; Franzin, B. T.; Pastre, I. A.; Fertonani, F. L. Thermal and kinetic studies of white lupin (Lupinus albus) oil. J. Therm. Anal. Calorim. 2018, 131 (1), 775–782. https://doi.org/10.1007/s10973-017-6468-0
Fontes, R. V.; Viana, A. P.; Pereira, M. G.; Oliveira, J. G.; Vieira, H. D. Manejo da cultura do híbrido de mamoeiro (Carica papaya L.) do grupo ‘Formosa’ UENF/CALIMAN: 01 para melhoria na qualidade do fruto com menor aplicação de adubação NPK. Rev. Bras. Frutic. 2012, 34 (1), 143–151. https://doi.org/10.1590/S0100-29452012000100020
Freire, P. C. M.; Mancini-Filho, J.; Ferreira, T. A. P. C. Principais alterações físico-químicas em óleos e gorduras submetidos ao processo de fritura por imersão: regulamentação e efeitos na saúde. Rev. Nutr. 2013, 26 (3), 353–358. https://doi.org/10.1590/S1415-52732013000300010
Friedman, H. L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C. 1964, 6 (1), 183–195. https://doi.org/10.1002/polc.5070060121
Hartman, L.; Lago, R. C. Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract. 1973, 22 (6), 475–476.
Jangle, R. D.; Magar, V. P.; Thorat, B. N. Phosphatidylcholine and its purification from raw de-oiled soya lecithin. Sep. Sci. Technol. 2013, 102, 187–195. https://doi.org/10.1016/j.seppur.2012.10.002
Jorge, N.; Soares, B. B. P.; Lunardi, V. M.; Malacrida, C. R. Physico-chemical alterations of sunflower, corn and soybean oils in deep fat frying. Quim. Nova. 2005, 28 (6), 947–951. https://doi.org/10.1590/S0100-40422005000600003
Judde, A.; Villeneuve, P.; Rossignol-Castera, A.; Le Guillou, A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J. Am. Oil Chem. Soc. 2003, 80 (12), 1209–1215. https://doi.org/10.1007/s11746-003-0844-4
Kalayasiri, P.; Jeyashoke, N.; Krisnagkurak, K. Survey of seed oils for use as diesel fuel. J. Am. Oil Chem. Soc. 1996, 73 (4), 471–474. https://doi.org/10.1007/BF02523921
Kardash, E.; Tur’yan, Y. I. Acid value determination in vegetable oils by indirect titration in aqueous-alcohol media. Croat. Chem. Acta. 2005, 78 (1), 99–103. https://hrcak.srce.hr/2797
Kobelnik, M.; Cassimiro, D. L.; Dias, D. S.; Ribeiro, C. A.; Crespi, M. S. Thermal behavior of jerivá oil (Syagrus romanzoffiana). J. Therm. Anal. Calorim. 2011, 106 (3), 711–715. https://doi.org/10.1007/s10973-011-1308-0
Kobelnik, M.; Cassimiro, D. L.; Dias, D. S.; Ribeiro, C. A.; Crespi, M. S. Thermal behavior of araca oil (Psidium cattleianum Sabine). J. Therm. Anal. Calorim. 2012, 108 (3), 1281–1286. https://doi.org/10.1007/s10973-011-1700-9
Kobelnik, M.; Fontanari, G. G.; Soares, R. A. M.; Figueiredo, A. G.; Ribeiro, C. A. Study of the thermal behavior of bicuíba oil (Virola bicuyba). J. Therm. Anal. Calorim. 2014, 115 (3), 2107–2113. https://doi.org/10.1007/s10973-013-3315-9
Kobelnik, M.; Fontanari, G. G.; Marques, M. R.; Ribeiro, C. A.; Crespi, M. S. Thermal behaviour and chromatographic characterization of oil extracted from the nut of the butia (Butia capitata). J. Therm. Anal. Calorim. 2016, 123 (3), 2517–2522. https://doi.org/10.1007/s10973-016-5239-7
Kobelnik, M.; Fontanari, G. G.; Ribeiro, C. A.; Crespi, M. S. Evaluation of thermal behavior and chromatographic characterization of oil extracted from seed of Pittosporum undulatum. J. Therm. Anal. Calorim. 2018a, 131 (1), 371–378. https://doi.org/10.1007/s10973-017-6763-9
Kobelnik, M.; Quarcioni, V. A.; Almeida, A. E.; Ribeiro, C. A.; Crespi, M. S. Study of the thermal behavior in solid state of Mn(II)-Diclofenac Complex. Eclét. Quím. 2018b, 43 (1), 59–66. https://doi.org/10.26850/1678-4618eqj.v43.1.59-66. https://doi.org/10.26850/1678-4618eqj.v43.1.2018.p59-66
Kobelnik, M.; Fontanari, G. G.; Soares, R. A. M.; Sampaio, G.; Ribeiro, C. A.; Crespi, M. S. Extraction of fatty acids contained in fruit from Ficus benjamina: lipid profile and thermal studies. J. Therm. Anal. Calorim. 2021, 146 (4), 1687–1693. https://doi.org/10.1007/s10973-020-10187-y
Lipp, M.; Simoneau, C.; Ulberth, F.; Anklam, E.; Crews, C.; Brereton, P.; Greyt, W.; Schwack, W.; Wiedmaier, C. Composition of genuine cocoa butter and cocoa butter equivalents. J. Food Comp. Anal. 2001, 14 (1), 399–408. https://doi.org/10.1006/jfca.2000.0984
Marques, M. R.; Fontanari, G. G.; Kobelnik, M.; Freitas, R. A. M. S.; Arêas, J. A. G. Effect of cooking on the thermal behaviour of the cowpea bean oil (Vigna unguiculata L. Walp). J. Therm. Anal. Calorim. 2015, 120 (1), 289–296. https://doi.org/10.1007/s10973-014-4125-4
Monetta, L. Uso da papaína nos curativos feitos pela enfermagem. Rev. Bras. Enferm. 1987, 40 (1) 66–73. https://doi.org/10.1590/S0034-71671987000100012
Patil, V. V.; Galge, R. V.; Thorat, B. N. Extraction and purification of phosphatidylcholine from soyabean lecithin. Sep. Sci. Technol. 2010, 75 (2), 138–144. https://doi.org/10.1016/j.seppur.2010.08.006
Puangsri, T.; Abdulkarim, S. M.; Ghazali, H. M. Properties of Carica papaya L. (papaya) seed oil following extractions using solvent and aqueous enzymatic methods. J. Food Lip. 2005, 12 (1), 62–76. https://doi.org/10.1111/j.1745-4522.2005.00006.x
Su, M. H.; Shih, M. C.; Lin, K-H. Chemical composition of seed oils in native Taiwanese Camellia species. Food Chem. 2014, 156, 369–373. https://doi.org/10.1016/j.foodchem.2014.02.016
Von Loesecke, H. W.; Notle, A. J. Characteristics and composition of papaya seed oil. J. Am. Chem. Soc. 1937, 59 (12), 2565–2567. https://doi.org/10.1021/ja01291a024
Xu, W.; Bai, W.; Guo, F.; Luo, Y.; Yuan, Y.; Huang, K. A papaya-specific gene, papain, used as an endogenous reference gene in qualitative and real-time quantitative PCR detection of transgenic papayas. Eur. Food Res. Technol. 2008, 228 (2), 301–309. https://doi.org/10.1007/s00217-008-0935-6

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Eclética Química