Abstract
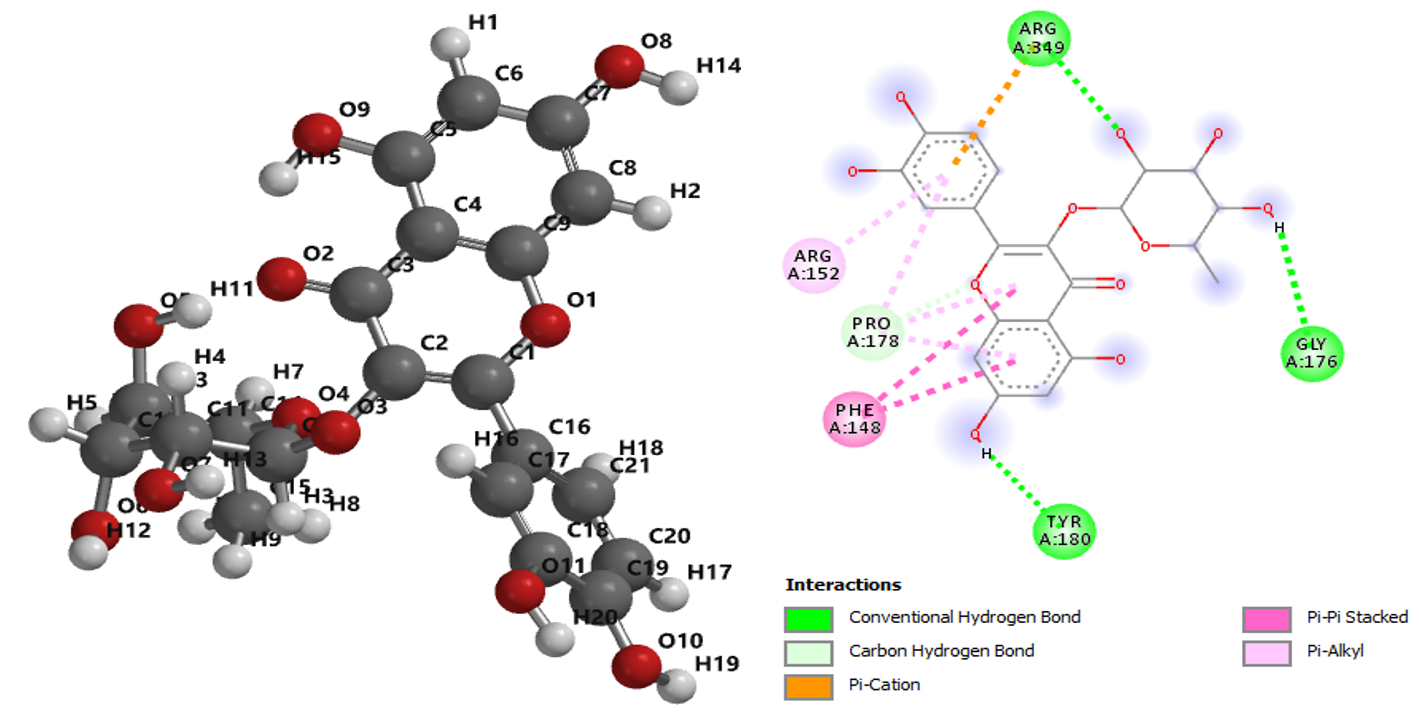
The use of medicinal plants as an alternative mean of treating various diseases has drawn the attention of several researchers. The desire to find lasting solutions to epilepsy among humans increases every day. Thus, this work was aimed at investigating the potential capacity of the studied phytochemicals in Alsophila spinulosa against human 4-aminobutyrate-aminotransferase as well as to predict the nonbonding interactions involved in the studied complexes. In this work, ten compounds with biological activities were selected and studied using molecular docking method. The molecules selected obtained from A. spinulosa leaves were optimized and various descriptors that described the anti-4-aminobutyrate-aminotransferase features were obtained. More so, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (compound 9) with highest binding affinity proved to have greater strength to inhibit 4-aminobutyrate-aminotransferase thereby downregulating epilepsy than other studied compounds and the reference drug (clobazam). The ADMET features of both compound 9 and clobazam were explored and reported.
References
Abbas, M.; Saeed, F.; Anjum, F. M.; Afzaal, M.; Tufail, T.; Bashir, M. S.; Ishtiaq, A.; Hussain, S.; Suleria, H. A. R. Natural polyphenols: An overview. Int J Food Prop. 2016, 20, 1689–1699. https://doi.org/10.1080/10942912.2016.1220393
Adeoye, M. D.; Oyebamiji, A. K.; Ashiru, M. A.; Adigun, R. A.; Olalere, O. H.; Semire, B. Biological evaluation of selected metronidazole derivatives as anti-nitroreductase via in silico approach. Eclét Quím. 2022, 47 (4), 27–36. https://doi.org/10.26850/1678-4618eqj.v47.4.2022.p27-36
Bartolini, E.; Ferrari, A. R.; Fiori, S.; Della Vecchia, S. Glycaemic imbalances in seizures and epilepsy of paediatric age: A literature review. J Clin Med. 2023, 12, 2580. https://doi.org/10.3390/jcm12072580
Chen, F.-Z.; Xiang, Q.-X.; Li, S.-H. Chemical constituents in the leaves of Alsophila spinulosa. Xibei Zhiwu Xuebao. 2008, 28 (6), 1246–1249.
Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P. W.; Tang, Y. AdmetSAR: a comprehensive source and free tool for evaluating chemical ADMET properties. J Chem Inf Model. 2012, 52 (11), 3099–3105. https://doi.org/10.1021/ci300367a
Chiang, H.-C.; Ying-Jui, L.; Fung-Jou, L. Xanthine oxidase inhibitors from the leaves of Alsophila spinulosa (HOOK) TRYON. J Enzyme Inhihitron. 1994, 8 (1), 61–71. https://doi.org/10.3109/14756369409040777
Chinese DmgDictionay, New Edition, (1985), Vol. 2, p. 1409, Fig. 2721. Sin Wen Hong Publications Inc., Taipei, Taiwan.
Choi, S.-Y.; Churchich, J. E. Biosynthesis of 4-aminobutyrate aminotransferase. Eur J Biochem. 1986, 161, 289–294. https://doi.org/10.1111/j.1432-1033.1986.tb10445.x
Dunkel, H.; Strzelczyk, A.; Schubert-Bast, S.; Kieslich, M. Facial emotion recognition in patients with juvenile myoclonic epilepsy. J Clin Med. 2023, 12, 4101. https://doi.org/10.3390/jcm12124101
Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar Tribe from Tamil Nadu, India. BMC Complement. Altern Med. 2006, 6. https://doi.org/10.1186/1472-6882-6-35
El fadili, M.; Er-Rajy, M.; Kara, M.; Assouguem, A.; Belhassan, A.; Alotaibi, A.; Mrabti, N.N.; Fidan, H.; Ullah, R.; Ercisli, S.; Zarougui, S.; Elhallaoui, M. QSAR, ADMET in silico pharmacokinetics, molecular docking and molecular dynamics studies of novel bicyclo (aryl methyl) benzamides as potent GlyT1 inhibitors for the treatment of schizophrenia. Pharmaceuticals. 2022a, 15, 670. https://doi.org/10.3390/ph15060670
El Fadili, M.; Er-Rajy, M.; Imtara, H.; Kara, M.; Zarougui, S.; Altwaijry, N.; Al Kamaly, O.; Al Sfouk, A.; Elhallaoui, M. 3D-QSAR, ADME-tox In Silico Prediction and molecular docking studies for modeling the analgesic activity against neuropathic pain of novel NR2B-selective NMDA receptor antagonists. Processes. 2022b, 10, 1462. https://doi.org/10.3390/pr10081462
Erazua, E. A.; Oyebamiji, A. K.; Akintelu, S. A.; Adewole, P. D.; Adelakun, A.; Adeleke, B. B. Quantitative Structure-Activity relationship, Molecular docking and ADMET screening of tetrahydroquinoline derivatives as anti-small cell lung cancer agents. Eclét Quím. 2023, 48 (1), 55–71. https://doi.org/10.26850/1678-4618eqj.v48.1.2023.p55-71
Gaglianoa, L.; Elie Bou, A.; Dang, K. N.; Sandy, R.; Mohamad, S. Bilateral preictal signature of phase-amplitude coupling in canine epilepsy. Epilepsy Res. 2018, 139, 123–128. https://doi.org/10.1016/j.eplepsyres.2017.11.009
Gao, X.; Jia, X.; Xu, M.; Xiang, J.; Lei, J.; Li, Y.; Lu, Y.; Zuo, S. Regulation of gamma-aminobutyric acid transaminase expression and its clinical significance in hepatocellular carcinoma. Front Oncol. 2022, 12, 879810. https://doi.org/10.3389/fonc.2022.879810
Hesdorffer, D. C.; Logroscino, G.; Benn, E. K.; Katri, N.; Cascino, G.; Hauser, W. A. Estimating risk for developing epilepsy: A population-based study in Rochester, Minnesota. Neurology. 2011, 76, 23–27. https://doi.org/10.1212/WNL.0b013e318204a36a
Hirtz, D.; Thurman, D. J.; Gwinn-Hardy, K.; Mohamed, M.; Chaudhuri, A. R.; Zalutsky R. How common are the ‘common’ neurologic disorders? Neurology. 2007, 68, 326–337. https://doi.org/10.1212/01.wnl.0000252807.38124.a3
Irene, C.-H.; Juan, R.-R.; Luis, G. B.-B.; Lilia, M.-L. Tree ferns (Cyatheaceae) as a source of phenolic compounds – A review. J Herb Med. 2022, 35, 100587. https://doi.org/10.1016/j.hermed.2022.100587
Jeżowska-Jurczyk, K.; Jurczyk, P.; Budrewicz, S.; Pokryszko-Dragan, A. Evaluation of event-related potentials in assessing cognitive functions of adult patients with epilepsy of unknown etiology. J Clin Med. 2023, 12, 2500. https://doi.org/10.3390/jcm12072500
Kan, W. S. Pharmaceutical botany. National Research Institute of Chinese Medicine, 1986.
Kebede, T.; Gadisa, E.; Tufa A. Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE. 2021, 16 (3), e0249253. https://doi.org/10.1371/journal.pone.0249253
Kim, K.; Yoon, H. Gamma-aminobutyric acid signaling in damage response, metabolism, and disease. Int J Mol Sci. 2023, 24, 4584. https://doi.org/10.3390/ijms24054584
Kwan, P.; Brodie, M. J. Early identification of refractory epilepsy. N Engl J Med. 2000, 342, 314–319. https://doi.org/10.1056/NEJM200002033420503
Lanza, K.; Gohlke, J.; Wang, S.; Sheffield, P. E.; Wilhelmi, O. Predicting the potential suitable habitats of Alsophila spinulosa and their changes. Int J Biometeorol. 2022, 66 (8), 1575–1588. https://doi.org/10.1007/s00484-022-02302-5
Longtine, C.; Tejedor A. Antimicrobial activity of ethanolic and aqueous extracts of medicinally used tree ferns Alsophila cuspidata and Cyathea microdonta. Acta Bot Malacit. 2017, 42 119–123. https://doi.org/10.24310/abm.v42i1.2885
Mishra, A.; Sharma A.; Kumar, S.; Saxena, A.; Pandey, A. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res Int. 2013, 2013, 1–10. https://doi.org/10.1155/2013/915436
Morton, C. V. Society the classification of the Cyatheaceae by R. M. Tryon. Am Fern J. 1971, 61, 142–143. https://doi.org/10.2307/1546647
Mukhopadhyay, H. K.; Kandar, C. C.; Das, S. K.; Ghosh, L.; Gupta, B. K. Epilepsy and its management: A review. Journal of PharmaSciTech. 2012, 1(2), 20-26.
Oyebamiji, A. K.; Akintayo, E. T.; Semire, B.; Odelade, K. A.; Adetuyi, B. O.; Amin, H.; Batiha, G. E. Insilico investigation on isatin(1H-indole-2,3-dione) derivatives as potential anti-tumor necrosis factor-alpha. Trop J Nat Prod Res. 2022, 6 (11), 1870–1875.
Oyeneyin, O. E.; Iwegbulam, C. G.; Ipinloju, N.; Olajide, B. F.; Oyebamiji, A. K. Prediction of the antiproliferative effects of some benzimidazolechalcone derivatives against MCF-7 breast cancer cell lines: QSAR and molecular docking studies. Org. Commun. 2022, 15 (3), 273–287. https://doi.org/10.25135/acg.oc.132.2203.2374
Pitout, J. D. D. Multiresistant Enterobacteriaceae: New threat of an old problem. Expert Rev Anti Infect Ther. 2008, 6 (5), 657–669. https://doi.org/10.1586/14787210.6.5.657
Poulakou, G.; Lagou, S.; Karageorgopoulos, D. E.; Dimopoulos, G. New treatments of multidrug-resistant Gram-negative ventilator-associated pneumonia. Ann Transl Med. 2018, 6 (20), 423–423. https://doi.org/10.21037/atm.2018.10.29
Queeny, I. P.; Daniel, C. M.; Jenny, C.; Robin, B. H.; David, M. L. An update on the prevalence and incidence of epilepsy among older adults. Epilepsy Res. 2018, 139, 107–112. https://doi.org/10.1016/j.eplepsyres.2017.11.022
Semire, B.; Oyebamiji, A. K.; Odunola O. Tailoring of Energy Levels in (2Z)-2-cyano-2-[2-[(E)-2-[2-[(E)-2-(p-tolyl)vinyl]thieno[3,2-b]thiophen-5-yl]vinyl]pyran-4-ylidene]acetic acid Derivatives via Conjugate Bridge and Fluorination of Acceptor units for Effective D-π-A Dye-Sensitized Solar Cells: DFT-TDDFT Approach. Res Chem Intermed. 2017, 43, 1863–1879. https://doi.org/10.1007/s11164-016-2735-0
Shen, S.; Butrin, A.; Beaupre, B. A.; Ferreira, G. M.; Doubleday, P. F.; Grass, D. H.; Zhu, W.; Kelleher, N. L.; Moran, G. R.; Liu, D.; Silverman, R. B. Structural and mechanistic basis for the inactivation of human ornithine aminotransferase by (3S,4S)-3-amino-4-fluorocyclopentenecarboxylic acid. Molecules. 2023, 28 (3), 1133. https://doi.org/10.3390/molecules28031133
Shriram, V.; Khare, T.; Bhagwat, R.; Shukla, R.; Kumar, V. Inhibiting bacterial drug efflux pumps via phytotherapeutics to combat threatening antimicrobial resistance. Front Microbiol. 2018, 9, 1–18. https://doi.org/10.3389/fmicb.2018.00001
Storici, P.; Biase, D.; Bossa, F.; Bruno, S.; Mozzarelli, A.; Peneff, C.; Silverman, R. B.; Schirmer, T. Structures of y-aminobutyric acid (GABA) aminotransferase, a pyridoxal 5’-phosphate, and [2Fe-2S] cluster-containing enzyme, complexed with y-ethynyl-GABA and with the antiepilepsy drug vigabatrin. J Biol Chem 2004, 279 (1), 363–373. https://doi.org/10.1074/jbc.M305884200
Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. https://doi.org/10.3390/microorganisms9102041
Vijayakumar, S.; Kasthuri, G.; Prabhu, S.; Manogar, P.; Parameswari, N. Screening and identification of novel inhibitors against human 4-aminobutyrate-aminotransferase: A computational approach. Egypt J Basic Appl Sci. 2018, 5, 210–219. https://doi.org/10.1016/j.ejbas.2018.05.008
Waziri, I.; Kelani, M. T.; Oyedeji-Amusa, M. O.; Oyebamiji, A. K.; Coetzee, L.-C. C.; Adeyinka, A. S.; Muller, A. J. Synthesis and computational investigation of N,N-dimethyl-4-[(Z)-(phenylimino)methyl] aniline derivatives: Biological and quantitative structural activity relationship studies. J Mol Struct. 2023, 1276, 134756. https://doi.org/10.1016/j.molstruc.2022.134756
Werhahn, K. J. Epilepsy in the elderly. Dtsch Arztebl Int. 2009, 106, 135–142. https://doi.org/10.3238/arztebl.2009.0135
Yan, N.; Zhang, H.; Zhang, Z.; Zhou, L.; Chen, T.; Feng, S.; Ding, C.; Yuan, M. The extraction, antioxidant and against b-amyloid induced toxicity of polyphenols from Alsophila spinulosa leaves. Arabian J Chem. 2022, 15, 103707. https://doi.org/10.1016/j.arabjc.2022.103707
Yasuhide, K.; Shigeko, F. S.; Koichi, M.; Tomoko, O.; Keiji, S.; Nanaya, T. The mature size of rat 4-aminobutyrate aminotransferase is different in liver and brain. Eur J Biochem. 1999, 264, 218–222. https://doi.org/10.1046/j.1432-1327.1999.00612.x
Zhang, M.; Zhong, H.; Cao, T.; Huang, Y.; Ji, X.; Fan, G. C.; Peng, T. Gamma-aminobutyrate transaminase protects against lipid overload-triggered cardiac injury in mice. Int J Mol Sci. 2022, 23 (4), 2182. https://doi.org/10.3390/ijms23042182

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Eclética Química