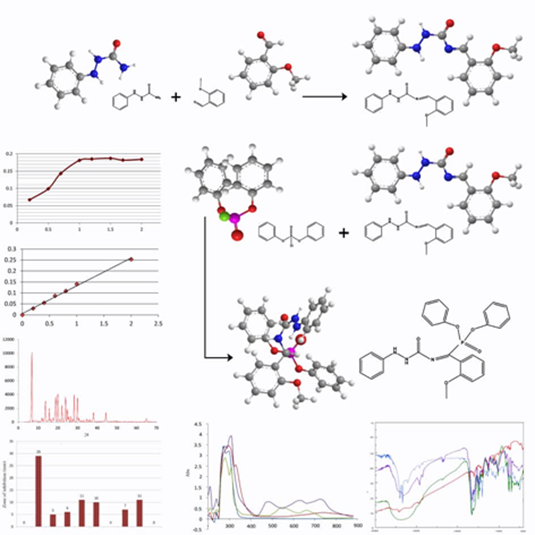
Abstract
Six complexes have been synthesized from Cu(II), Ni(II), and Co(II) with new bidentate N2 donor Schiff base ligand (2-methoxybenzalidene-1-phenylsemicarbazide L1) and tridentate N2O donor organophosphorus Schiff base ligand (2-methoxybenzalidenediphenylphosphate-1-phenylsemicarbazide L2). Both ligands were synthesized and characterized by metal analysis, infrared (IR), ultraviolet visible (UV-Vis), and nuclear magnetic resonance (NMR) spectral studies. The chemical structures of the synthesized complexes were characterized using their metal analysis, magnetic susceptibility, molar conductance, IR, and UV-Vis spectra. According to molar ratio studies, the complexes have the composition of ML2 for L1 and ML for L2. The X-ray diffraction (XRD) studies showed that the particle size of ligands and L1 complexes were in nano-range. The ligands and their metal complexes have been screened for their antioxidant, antibacterial and antifungal activity.
References
Abd El-Wahab, Z. H.; El-Sarrag, M. R. Derivatives of phosphate Schiff base transition metal complexes: synthesis, studies and biological activity. Spectrochimica Acta Part A. 2004, 60, 271–277. https://doi.org/10.1016/S1386-1425(03)00216-6
Abu-Dief, A. M.; El-Metwaly, N. M.; Alzahrani, S. O.; Bawazeer, A. M.; Shaaban S.; Adam, M. S. S. Targeting ctDNA binding and elaborated in-vitro assessments concerning novel Schiff base complexes: Synthesis, characterization, DFT and detailed in-silico confirmation. J. Mol. Liq. 2021, 322, 114977. https://doi.org/10.1016/j.molliq.2020.114977
Abu-Yamin, A.; Abduh, M. S.; Saghir, S. A. M.; Al-Gabri, N. Synthesis, characterization and biological activities of new Schiff base compound and its lanthanide complexes. Pharmaceuticals 2022, 15 (4), 454. https://doi.org/10.3390/ph15040454
Aderoju, A. O.; Sherifah, M. W. Synthesis, characterization and antimicrobial activity of some mixed drug trimethoprim-sulfamethoxazole metal drug complexes. World Appl. Sci. J. 2015, 33 (2), 336–342. https://doi.org/10.5829/idosi.wasj.2015.33.02.22206
Akhtar M. S.; Alenad, A.; Malik, M. A. Synthesis of mackinawite FeS thin films from acidic chemical baths. Mater. Sci. Semicond. Process. 2015, 32, 1–5. https://doi.org/10.1016/j.mssp.2014.12.073
Al-Azab, F. M.; Jamil, Y. M. S.; Al-Gaadbi,A. A. M., Synthesis and Spectroscopic methods on the Complexation of CoII, NiII and CuII with 2-(((1H-indol-3-yl) methylene) amino) acetohydrazide hydrate. Sana’a University J. Appl. Sci. Technol. 2023, 1 (1), 117–133. https://doi.org/10.59628/jast.v1i1.131
Al-Hakimi, A. N.; Shakdofa, M. M. E.; El-Seidy, A. M. A.; El-Tabl, A. S. Synthesis, Spectroscopic, and biological studies of chromium(iii), manganese(ii), iron(iii), cobalt(ii), nickel(ii), copper(ii), ruthenium(iii), and zirconyl(ii) complexes of N1,N2-Bis(3-((3-hydroxynaphthalen-2-yl)methylene-amino)propyl)phthalamide. J. Korean Chem. Soc. 2011, 55 (3), 418–429. https://doi.org/10.5012/jkcs.2011.55.3.418
Al-Maydama, H.; Al-Ansi, T. Y.; Jamil, Y. M.; Ali, A. H. Biheterocyclic ligands: synthesis, characterization and coordinating properties of bis(4-amino-5-mercapto-1,2,4-triazol-3-yl)alkanes with transition metal ions and their thermokinetic and biological studies. Eclét. Quím. 2008, 33 (3), 29–42. https://doi.org/10.1590/S0100-46702008000300005
Boverhof, D. R.; Bramante, C. M.; Butala, J. H.; Clancy, S. F.; Lafranconi, M.; West, J.; Gordon, S. C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73 (1), 137–150. https://doi.org/10.1016/j.yrtph.2015.06.001
Brzezińska-Błaszczyk, E.; Mińcikiewicz, M.; Ochocki, J. Effect of cisplatin and cis-platinum (II) phosphonate complex on murine mast cells. Eur. J. Pharmacol. 1996, 298 (2), 155–158. https://doi.org/10.1016/0014-2999(95)00809-8
Çakır, U.; Temel, H.; Ihan, S.; Uğraş, H. I. Spectroscopic and conductance studies of new transition metal complexes with a Schiff base derived from 4-methoxybenzaldehyde and 1,2-bis(p-aminophenoxy)ethane. Spectrosc. Lett. 2003, 36 (5–6), 429–440. https://doi.org/10.1081/SL-120026609
Camellia, F. K.; Ashrafuzzaman, M.; Islam, M. N.; Banu, L. A.; Kudrat-E-Zahan, M. Isoniazid Derived Schiff Base Metal Complexes: Synthesis, Characterization, Thermal Stability, Antibacterial and Antioxidant Activity Study. Asian J. Chem. Sci. 2022, 11 (4), 23-36. https://doi.org/10.9734/ajocs/2022/v11i419131
Carrasco, F.; Hernández, W.; Chupayo, O.; Álvarez, C. M.; Oramas-Royo, S.; Spodine, E.; Tamariz-Angeles, C.; Olivera-Gonzales, P.; Dávalos, J. Z. Indole-3-carbaldehyde semicarbazone derivatives: Synthesis, characterization and antibacterial activities. J. Chem. 2020, 2020, 7157281. https://doi.org/10.1155/2020/7157281
Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M. S. A Review on the antimicrobial activity of Schiff bases: data collection and recent studies. Antibiotics 2022, 11 (2), 191. https://doi.org/10.3390/antibiotics11020191
El-khazandar, A. N. Organo-phosphorus Schiff base part (IV): Synthesis and characteristic of some phosphate Schiff-base complexes. Phosphorus Sulfur Silicon Relat. Elem. 1997, 126 (1), 243–255. https://doi.org/10.1080/10426509708043564
El-Tabl, A. S.; El-Saied, F. A.; Al-Hakimi, A. N. Synthesis, spectroscopic investigation and biological activity of metal complexes with ONO trifunctionalalized hydrazone ligand. Transition Met. Chem. 2007, 32 (6), 689–701. https://doi.org/10.1007/s11243-007-0228-0
Ericsson, H.; Tunevall, G.; Wickman, K. The Paper Disc Method for Determination of Bacterial Sensitivity to Antibiotics: Relationship Between the Diameter of the Zone of Inhibition and the Minimum Inhibitory Concentration. Scand. J. Clin. Lab. Invest. 1960, 12 (4), 414–422. https://doi.org/10.3109/00365516009065406
Fouda, M. F. R.; Abd-Elzaher, M. M.; Shakdofa, M. M.; El-Saied, F. A.; Ayad, M. I.; El Tabl, A. S. Synthesis and characterization of a hydrazone ligand containing antipyrine and its transition metal complexes. J. Coord. Chem. 2008, 61 (12), 1983–1996. https://doi.org/10.1080/00958970701795714
Franklin, T. J.; Snow, G. A. Biochemistry of Antimicrobial Action; Springer, 1989. https://doi.org/10.1007/978-94-009-0825-3
Galil, M. S. A.; Al-Hakimi, A. N.; Alshwafy, R. Y.; Al Okab, R. A.; Mutir, A. Synthesis, Structural Studies and Microbial Evaluation of Cu(II), Mn(II) Ni(II), Zn(II), Fe(III), Ru(III), VO(II), UO2(II) Complexes of Tetradentate Oxime-Hydrazon Ligand. Chem. J. 2015, 1 (3), 95–102.
Geary, W. J. The use of conductivity measurements in organic solvents for characterization of coordination compounds. Coord. Chem. Rev. 1971, 7 (1), 81–122. https://doi.org/10.1016/S0010-8545(00)80009-0
González-García, C.; Mata, A.; Zani, F.; Mendiola M. A.; López-Torres, E. Synthesis and antimicrobial activity of tetradentate ligands bearing hydrazone and/or thiosemicarbazone motifs and their diorganotin (IV) complexes. J. Inorg. Biochem. 2016, 163, 118–130. https://doi.org/10.1016/j.jinorgbio.2016.07.002
Gup, R.; Kirkan, B. Synthesis and spectroscopic studies of copper(II) and nickel(II) complexes containing hydrazonic ligands and heterocyclic colig and Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 62 (4–5), 1188–1195. https://doi.org/10.1016/j.saa.2005.04.015
Hossain, M. S.; Camellia, F. K.; Uddin, N.; Kudrat-E-Zahan, M.; Banu, L. A.; Haque, M. M. Synthesis, Characterization and Antimicrobial Activity of Metal Complexes of N-(4-methoxybenzylidene) Isonicotinohydrazone Schiff Base. Asian J. Chem. Sci. 2019, 6 (1), 1–8. https://doi.org/10.9734/ajocs/2019/v6i118987
Ibeji, C. U.; Akintayo, D. C.; Oluwasola, H. O.; Akintemi, E. O. Anti-Corrosion potential of the Ortho and ParaSubstituted Schiff Bases of 2-Methoxybenzaldehyde on Fe (110) surface in acid medium: Synthesis, DFT and Molecular Dynamics Studies. Research square. 2022. [Preprint]. https://doi.org/10.21203/rs.3.rs-1869552/v1
Jamil, Y.M.S.; Al-Azab, A. M.; Al-Azab, F. M.; Al-Selwi, N. A. A. Larvicidal Effects of New Organophosphorus Schiff base compounds against Dengue Fever Vector Aedes aegypti (Diptera; Culicidae), Sana’a University J. Appl. Sci. Technol. 2023, 1 (1), 78–87. https://doi.org/10.59628/jast.v1i1.156
Jassem, A. M.; Radhi, W. A.; Jaber, H. A.; Mohammed, F. J. Synthesis and characterization of 1,3,4-Oxadiazoles Derivatives from 4-Phenyl-Semicarbazide. J. Basrah Res. Sci. 2013, 39 (3), 158–170.
Kaczmarek, M. T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. https://doi.org/10.1016/j.ccr.2018.05.012
Kafi-Ahmadi, L.; Marjani, A. P. Mononuclear Schiff Base Complexes Derived from 5-Azophenylsalicylaldehyde with Co(II), Ni(II) Ions: Synthesis, Characterization, Electrochemical Study and Antibacterial Properties. S. Afr. J. Chem. 2019, 72 (1), 101–107. https://doi.org/10.17159/0379-4350/2019/v72a13
Kostova, I.; Saso, L. Advances in Research of Schiff-Base Metal Complexes as Potent Antioxidants. Curr. Med. Chem. 2013, 20 (36), 4609–4632. https://doi.org/10.2174/09298673113209990149
Mohammed, N. L.; Al-Shawi, J. M. S.; Kadhim, M. J. Synthesis, Characterization and Thermal Studies of Schiff Bases Derived from 2,4-Dihydroxy benzaldehyde and their Complexes with Co(II), Ni (II), Cu(II). Int. J. Sci. Eng. Res. 2019, 7 (1), 31–40.
Mohapatra, R. K.; Mishra, U. K.; Mishra, S. K.; Mahapatra, A.; Dash, D. C. Synthesis and Characterization of Transition Metal Complexes with Benzimidazolyl-2-hydrazones of o-anisaldehyde and Furfural. J. Korean Chem. Soc. 2011, 55 (6), 926–931. https://doi.org/10.5012/jkcs.2011.55.6.926
Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A and Part B; John Wiley & Sons, 1998.
Ochocki, J.; Graczyk, J.; Reedijk, J. Synthesis and antitumor activity of novel Pt(II) diethyl pyridylmethylphosphonate complexes against sarcoma-180. J. Inorg. Biochem. 1995, 59 (2–3), 240. https://doi.org/10.1016/0162-0134(95)97346-R
Patterson, A. L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56 (10), 978–982. https://doi.org/10.1103/PhysRev.56.978
Sani, U.; Iliyasu, S. M. Synthesis, characterization and antimicrobial studies on Schiff base derived from 2-aminopyridine and 2-methoxybenzaldehyde and its cobalt (II) and nickel (II) complexes. Bayero J. Pure appl. Sci. 2018, 11 (1), 214–219. https://doi.org/10.4314/bajopas.v11i1.35S
Shah, B.; Kakumanu, V. K.; Bansal, A. K. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J. Pharm. Sci. 2006, 95 (8), 1641–1665. https://doi.org/10.1002/jps.20644
Shakdofa, M. M. E.; Al-Hakimi, A. N.; Elsaied, F. A.; Alasbahi, S. O. M.; Alkwlini, A. M. A. Synthesis, Characterization and Bioactivity Zn2+, Cu 2+, Ni2+, Co2+, Mn2+, Fe3+, Ru3+, VO2+ and UO2+ complexes of 2-hydroxy-5-((4-nitrophnyl(dizenyl)benzylidene)-2-(p-tolylamino)acetohydrazide. Bull. Chem. Soc. Ethiop. 2017, 31 (1), 75–91. https://doi.org/10.4314/bcse.v31i1.7
Song, X.-Q.; Wang, Z.-G.; Wang, Y.; Huang, Y.-Y.; Sun, Y.-X.; Ouyang, Y.; Xie, C.-Z.; Xu, J.-Y. Syntheses, characterization, DNA/HSA binding ability and antitumor activities of a family of isostructural binuclear lanthanide complexes containing hydrazine Schiff base. J. Biomol. Struct. Dyn. 2020, 38 (3), 733–743. https://doi.org/10.1080/07391102.2019.1587511
Vogel, A. I. Text-book of quantitative inorganic analysis including elementary instrumental analysis; Longmans, 1961.
Yassin, S. K.; Alshawi J. M. S.; Salih, Z. A. M. Synthesis, characterization and cytotoxic activity study of Cu (II), Co (II), Mn (II), Ni (II) and Cr (III) Metal Complexes with new guanidine Schiff base against the hepatocellular Carcinoma (HCAM) cancer cell. Egypt. J. Chem. 2020, 63 (10), 4005-4016. https://doi.org/10.21608/ejchem.2020.37893.2778
Yoe, J. H.; Jones, A. L. Colorimetric Determination of Iron with Disodium-1,2-dihydroxybenzene-3,5-disulfonate. Ind. Eng. Chem. Anal. Ed. 1944, 16 (2), 111–115. https://doi.org/10.1021/i560126a015
Yusof, E. N. M.; Ravoof, T. B. S. A.; Tiekink, E. R. T.; Veerakumarasivam, A.; Crouse, K. A.; Tahir, M. I. M.; Ahmad, H. Synthesis, Characterization and Biological Evaluation of Transition Metal Complexes Derived from N, S Bidentate Ligands. Int. J. Mol. Sci. 2015, 16 (5), 11034–11054. https://doi.org/10.3390/ijms160511034

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Eclética Química