Abstract
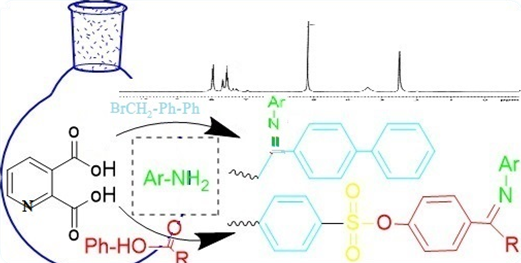
In this paper, some substituted imines compounds have been prepared from quinolinic acid as a starting material. Firstly, the quinolinic acid was treated with acetic anhydride and acetic acid to form furo[3,4-b]pyridine-5,7-dione (1); the resulting compound was heated with urea to form 5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione (2). After that, it was treated with potassium hydroxide to give potassium 5,7-dioxo-5,7-dihydropyrrolo[3,4-b]pyridin-6-dione, which was directly and easily converted to 6-(2-([1,1'-biphenyl]-4-yl)-2-oxoethyl)-5H-pyrrolo[3,4-b]pyridine-5,7(6H)-dione (3) by the reaction with 1-([1,1'-biphenyl]-4-yl)-2-bromoethan-1-one. Finally, the resultant compound reacted with substituted aniline to give imines (4, 5). Secondly the quinolinic acid converted to 4-(5,7-dioxo-5,7-dihydro-6H-pyrrolo[3,4-b] pyridin-6-yl) benzenesulfonyl chloride according to our previous work, then treated with p-hydroxy acetophenone or p-hydroxy benzaldehyde to form 4-substituted bezyloxy 4-(5,7-dioxo-5,7-dihydro-6H-pyrrolo[3,4-b] pyridine-6-yl) benzenesulfonate (6, 7), which were finally treated with substituted aniline to form new substituted imines (8–12).
References
Aliabadi, A.; Foroumadi, A.; Mohammadi-Farani, A; Mahvar, M. G. Synthesis and Evaluation of Anti-acetylcholinesterase Activity of 2-(2-(4-(2-Oxo-2-phenylethyl) piperazin-1-yl) ethyl)Isoindoline-1,3-dione Derivatives with Potential Anti-Alzheimer Effects. Iran J. Basic Med. Sci. 2013, 16 (10), 1049–1054.
Altaee, E. A.; Al-Sabawi, A. H. Synthesis and Spectral Study of Some New 4-substituted but-2-enolide Derivatives. Egypt. J. Chem. 2021, 64 (12), 7117–7122. https://doi.org/10.21608/ejchem.2021.80154.3957
Bashiri, M.; Jarrahpour, A.; Rastegari, B.; Iraji, A.; Irajie, C.; Amirghofran, Z.; Malek-Hosseini, S.; Motamedifar, M.; Haddadi, M.; Zomorodian, K.; Zareshahrabadi, Z.; Turos, E. Synthesis and evaluation of biological activities of tripodal imines and β-lactams attached to the 1,3,5-triazine nucleus. Monatsh Chem. 2020, 151 (5), 821–835. https://doi.org/10.1007/s00706-020-02592-8
Cai, Y.-H. Solvent-Free Synthesis of Phthalimide Under Microwave Irradiation and Modification of Talc with Synthesized Phthalimide. Asian J. Chem. 2012, 24 (2), 481–484.
Chan, K. K.; Wong, Y. F.; Yang, D.; Pettus, T. R. R. Nucleophilic Imines and Electrophilic o-Quinone Methides, a Three-Component Assembly of Assorted 3,4-Dihydro-2H-1,3-benzoxazines. Org. Lett. 2019, 21 (19), 7746–7749. https://doi.org/10.1021/acs.orglett.9b02655
Choudhury, L. H.; Parvin, T. Recent advances in the chemistry of imine-based multicomponent reactions (MCRs). Tetrahedron, 2011, 67 (43), 8213–8228. https://doi.org/10.1016/j.tet.2011.07.020
Fadlelmoula, A.; Pinho, D.; Carvalho, V. H.; Catarino, S. O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines, 2022, 13 (2), 187. https://doi.org/10.3390/mi13020187
Fathi, A. A.; Al-Jawaheri, Y. S. M. Synthesis and characterization of new N- Aryl sulfonyl hydrazone compounds. Egypt. J. Chem. 2022, 65 (3), 179–183. https://doi.org/10.21608/ejchem.2021.90637.4320
Hania, M. M. Synthesis of Some Imines and Investigation of their Biological Activity. J. Chem. 2009, 6 (3), 629–632. https://doi.org/10.1155/2009/104058
Jasril, J.; Ikhtiarudin, I.; Nurulita, Y.; Nurisma. Microwave-assisted synthesis and antioxidant activity of an imine, (E)-1-(3-bromobenzylidene)-2-phenylhydrazine. AIP Conf. Proc. 2020, 2242, 040041. https://doi.org/10.1063/5.0009374
Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff Bases: A Versatile Pharmacophore. J. Catal. 2013, 2013, 893512. https://doi.org/10.1155/2013/893512
Maimaris, M.; Pettipher, A. J.; Azzouzi, M.; Walke, D. J.; Zheng, X.; Gorodetsky, A.; Dong, Y.; Tuladhar, P. S.; Crespo, H.; Nelson, J.; Tisch, J. W. G.; Bakulin, A. A. Sub-10-fs observation of bound exciton formation in organic optoelectronic devices. Nat. Commun. 2022, 13, 4949. https://doi.org/10.1038/s41467-022-32478-8
Rapolu, R. K.; Areveli, S.; Raju, V. V. N. K. V. P.; Navuluri, S.; Chavali, M.; Mulakayala, N. An Efficient Synthesis of Darunavir Substantially Free from Impurities: Synthesis and Characterization of Novel Impurities. ChemistrySelect. 2019, 4 (14), 4422–4427. https://doi.org/10.1002/slct.201803825
Silva, E. T.; Araújo, A. S.; Moraes, A. M.; Souza, L. A.; Lourenço, M. C. S.; Souza, M. V. N.; Wardell, J. L.; Wardell, S. M. S. V. Synthesis and Biological Activities of Camphor Hydrazone and Imine Derivatives. Sci. Pharm. 2016, 84 (3), 467–483. https://doi.org/10.3390/scipharm84030467
Soyer, Z.; Uysal, S.; Parlar, S.; Dogan, A. H. T.; Alptuzun, V. Synthesis and molecular docking studies of some 4-phthalimidobenzenesulfonamide derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32 (1), 13–19. https://doi.org/10.1080/14756366.2016.1226298
Yin, Y.; Zhao, X.; Jiang, Z. Advances in the Synthesis of Imine-Containing Azaarene Derivatives via Photoredox Catalysis. ChemCatChem. 2020, 12 (18), 4471–4489. https://doi.org/10.1002/cctc.202000741

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Eclética Química