Abstract
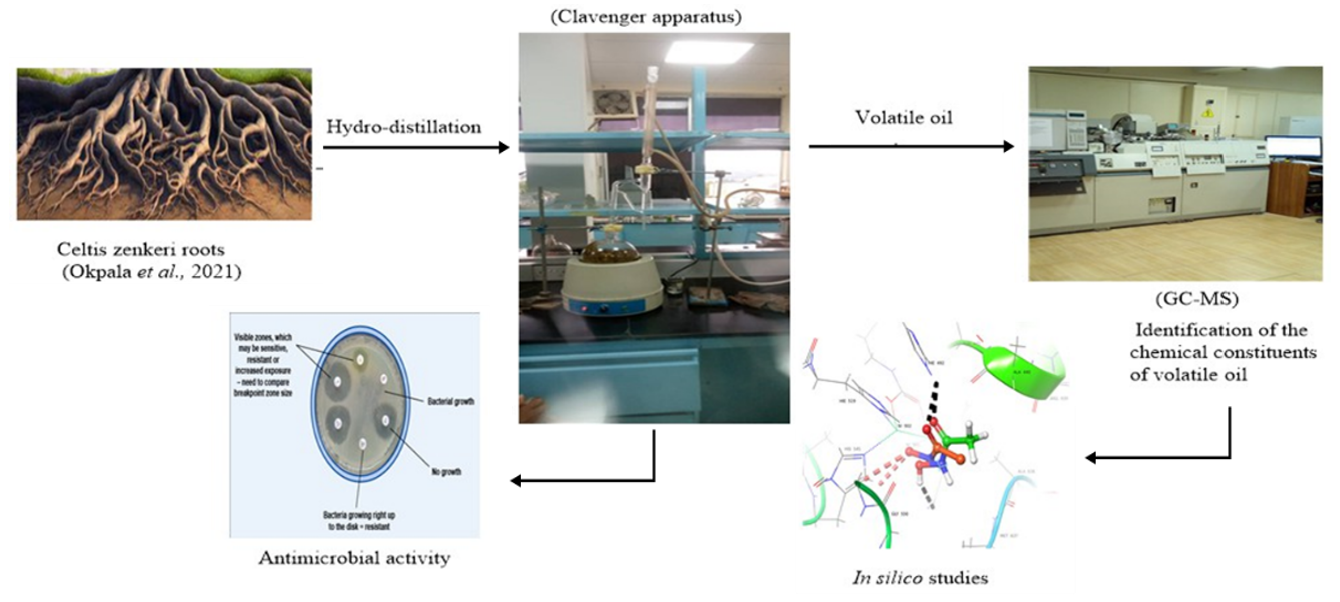
This study is aimed at investigating the volatile constituents of the air-dried roots of Celtis zenkeri. The volatile oil was extracted using hydro-distillation method and characterised using gas chromatography-mass spectrometry (GC-MS). The volatile oil was screened against six selected bacteria and four fungi strains using the agar diffusion method. The molecular docking study of the identified compounds was conducted to investigate their binding pattern with the substrate and nucleotide complexes of Enterococcus faecium aminoglycoside-2’’-phosphotransferase-IIa [APH(2’’)-IIa] (PDB ID: 3HAM) and full-length Lanosterol 14 alpha-Demethylases of Prominent fungal pathogens Candida albicans (PDB ID: 5V5Z). The yield of the volatile oil (% w/w) root of C. zenkeri was 0.79%. Six compounds were identified in the root essential oil representing 80.07% of the volatile oil. 2-methyl-1-pentene (40.01%) was the most abundant compound in the root essential oil. The volatile oil from roots of the C. zenkeri exhibited good activity against all the screened bacteria and fungi strains at a concentration of 12.5-100 mg/mL when compared with Gentamicin for bacteria and Tioconazole for fungi.
References
Adepoju, A. J.; Latona, D. F.; Olafare, O. G.; Oyebamiji, A. K.; Abdul-Hammed, M.; Semire, B. Molecular docking and pharmacokinetics studies of Curcuma longa (Curcumin) potency against Ebola virus. Ovidius Univ. Ann. Chem. 2022, 33 (1), 22–35. https://doi.org/10.2478/auoc-2022-0004
Ata, A.; Van Den Bosch, S. A.; Harwannik, D. J.; Pidsinski, G. E. Gluthathione-S transferase and acetylcholinesterase inhibiting natural products from medicinally important plants. Pure Appl. Chem. 2007, 70, 2269–2279. https://doi.org/10.1351/pac200779122269
Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils- a review. Food Chem. Toxicol. 2008, 46 (2), 446–4475. https://doi.org/10.1016/j.fct.2007.09.106
Bottomley, M. J.; Muraglia, E.; Bazzo, R.; Carfì, A. Molecular Insights into Quorum Sensing in the Human Pathogen Pseudomonas aeruginosa from the Structure of the Virulence Regulator LasR Bound to Its Autoinducer. J. Biol. Chem. 2007, 282 (18), 13592–13600. https://doi.org/10.1074/jbc.m700556200
Paterson, G. R. (1982). British Pharmacopoeia 1980. Can. Med. Assoc. J. 1982, 126 (5), 514.
Burkill, H. M. The useful plants of West tropical Africa. Royal Botanic garden Kew. 1995, 2, 160–163.
Casigilia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini D.; Maggi, F. Kundmannia sicula (L.) DC: a rich source of germacrene D. J. Essent. Oil Res. 2017, 29 (6) 437-442. https://doi.org/10.1080/10412905.2017.1338625
Cassel, E.; Vargas R. M. F. Experiments and modeling of the Cymbopogon winterianus essential oil extraction by steam distillation. J. Mexican Chem. Soc. 2006, 50, 126–129.
Di Leo Lira, P.; Retta, D.; Tkacik, E.; Ringuelet, J.; Coussio, J. D.; Van Baren, C.; Bandoni A. L. Essential oil and by-products of distillation of bay leaves (Laurusnobilis L.) from Argentina. Ind. Crops Prod. 2009, 30 (2), 259–264. https://doi.org/10.1016/j.indcrop.2009.04.005
El-Mahmood, A. M.; Doughari, J. H. Phytochemical Screening and Antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. Afr. J. Pharm. Pharmacol. 2008, 2 (7), 124–129.
Fabeku, P. O. Traditional Medicine: the art, ways and practice. In: Odugbemi, T. (Ed.). Outlines and Pictures of Medicinal Plants from Nigeria; University of Lagos Press, 2006, p. 13–24.
Fadipe, A. L. Some fatty acids ester of the ripe fruits of Nauclea latifolia (family: Rubiacea). Inter. J. Res. Pharm. Chem. 2014, 4 (4), 783–788.
Hamburger, M.; Hostettmann, K. Bioactivity in plants: the link between phytochemistry and medicine. Phytochemistry. 1991, 30 (12), 3864–3874. https://doi.org/10.1016/0031-9422(91)83425-K
Hanazki, N.; Tamishoro, J. Y.; Leitao-Filho, H.; Gegossi, A. Diversity of Plant use in Caicaras Communities from the Atlantic forest coast, Brazil. Biodiversity and Conservation. 2000, 9, 597–615.
Ibok, M. G.; Odeja, O. O.; Okpala, E. O.; Eghwubare, J. E.; Anifalaje E. O. Eremomastax speciosa (Hochst.): GC/MS Profiling, Antioxidant and Antimicrobial Activities of Stem Essential oil. Futur. J. Pharm. Sci. 2023, 9, 51. https://doi.org/10.1186/s43094-023-00501-4
Janssen, A. M.; Scheffer, J. J. C.; Baerheim-Svendsen, A. Antimicrobial activities of essential oils. A 1976-1986 literature review on possible applications. Pharm. Weekblad Sci. Edu. 1987, 9, 193–197. https://doi.org/10.1007/BF02029329
Kaur, S.; Singh, H. P.; Batish, D. R.; Kohli, R. K. Chemical characterization, antioxidant and antifungal activity of essential oil from Eucalytus tereticornis. Journal of medicinal plants Research. 2011, 5 (19), 4788–4793.
Kiashi, F.; Momeni-nasab, F.; Akhbar, M.; Hadjiakhoondi, A.; Aghaahmeddi M.; Tavakoli S.; Tofighi Z. Phytochemicals and antimicrobial activities of aerial parts and roots of Trigonella tehranica L. essential oils. Res. J. Pharmacogn. 2017, 4 (4) 29–30.
Lapa, G. B.; Bekker, O. B.; Mirchink, E. P.; Danilenko, V. N.; Preobrazhenskaya, M. N. Regioselective acylation of congeners of 3-amino-1H-pyrazolo[3,4-b]quinolines, their activity on bacterial serine/threonine protein kinases and in vitro antibacterial (including antimycobacterial) activity. J. Enzyme Inhib. Med. Chem. 2012, 28 (5), 1088–1093. https://doi.org/10.3109/14756366.2012.716056
Lima-Filho, J. V. M.; Carvalho, A. F. F. U.; Freitas, S. M.; Melo, V. M. M. Antibacterial activity of extracts of six macroalgae from the North-eastern Brazillian coast. Braz. J. Microbiol. 2002, 33 (4), 311–313. https://doi.org/10.1590/S1517-83822002000400006
Masango, P. Cleaner production of essential oils by steam distillation. J. Cleaner Prod. 2005, 13 (8) 833–839. https://doi.org/10.1016/j.jclepro.2004.02.039
Mohamed, A. A.; El-Emary, G. A.; Ali, H. F. Influence of some citrus essential oils on cell viability, glutathione-s-transferase and lipid peroxidation in Ehrlich ascites Carcinoma cells. J. Am. Sci. 2010, 6, 820–826.
Mockute, D.; Bernotiene, G.; Judzentiene, A. The essential oils with dominant germacrene D of Hypericum perforatum L. growing wild in Lithuania. J. Essent. Oil Res. 2008, 20 (2), 128–131. https://doi.org/10.1080/10412905.2008.9699973
Narramore, S.; Stevenson, C. E.; Maxwell, A.; Lawson, D. M.; Fishwick, C. W. New insights into the binding mode of pyridine-3-carboxamide inhibitors of E. coli DNA gyrase. Bioorganic & Medicinal Chemistry. 2019, 27 (16), 3546–3550. https://doi.org/10.1016/j.bmc.2019.06.015
Nazemi, M.; Motallebi A.; Abbasi Z.; Khaledi M.; Zare, M. Antibacterial, antifungal and cytotoxic activity of the fraction contains squalene in the acetone extract of a sea cucumber, Stichopus hermanni. Iran. J. Fish. Sci. 2022, 21 (6) 1495–1507.
Neumann, R. R.; Hirsch, E. Commercialization of Non-Timber Forest Products: Review and Analysis for Research; CIFOR, 2000.
Ngoupayo, J.; Kasali F. M.; Djiele N. P.; Turibio T. K.; Ali, M. S. Antimicrobial of extract and compounds from the bark of Drypetes afzelii (pax) Hutch. J. Pharmacogn. Phytochem. 2015, 4 (4), 250–255.
Noriege P.; Guerrini, A.; Sacchetti G.; Grandini, A.; Ankuash E.; Manfredini, S. Chemical composition and biological activity of five essential oils from Ecuadorian Amazon rain forest. Molecule. 2019, 24 (8), 1637. https://doi.org/10.3390/molecules24081637
Obame, L. C.; Edou P.; Bassole I. H. N.; Koudou, J.; Agnaniet, H.; Eba, F.; Traore, A. S. Chemical composition, antioxidant and antimicrobial properties of essential oil of Dacryodes edulis (G. Don) H. J. Lam from Gabon. African J. Microbiol. Res. 2008, 2, 146–152.
Odeja O. O.; Okpala E. O.; Ibok, M. G.; Okoro, E. E.; Onoja J. O. Essential oil Composition, Antioxidant and Antibacterial Activities of Jatropha tanjorensis (Euphorbiaceae). Ann. Rev. Resear. 2023, 9 (1), 55575. https://doi.org/10.19080/arr.2023.09.555751
Ogunnusi, T. A.; Oso, B. A.; Dosumu, O. O. Isolation and antibacterial activity of triterpeme from Euphorbia kamerunica pax. Int. J. Biol. Chem. Sci. 2010, 4 (1), 158–167. https://doi.org/10.4314/ijbcs.v4i1.54241
Okpala, E. O.; Oloyede, G. K.; Onocha, P. A. Chemical Composition, Antimicrobial and Antioxidant Activities of Volatile oil of Euphorbia graminea JACQ from Nigeria. Int. J. Adv. Sci. Eng. Inf. Techno. 2019, 7 (4), 50–54.
Okpala, E. O.; Onocha, P. A.; Ali, M. S.; Zikr-Ur-Rehmen, S.; Lateef, M. Zenkeramide: a new iso-benzofuranone propanamide and urease inhibitory constituents of Celtis zenkeri Engl stem bark (Ulmaceae). Nat. Prod. Res. 2021, 37 (1) 93–98. https://doi.org/10.1080/14786419.2021.1954643
Okpala, E. O.; Onocha, P. A.; Ali, M. S. Antioxidant activity of phytol dominated stem bark and leaf essential oils of Celtis zenkeri Engl. Trends Phytochem. Res. 2022, 6 (2), 137–144. https://doi.org/10.30495/tpr.2022.1952985.1246
Olaoluwa, O. O.; Olapeju, A. O. Phytochemical investigation and antimicrobial screening of Cardiospermun grandiflorum (Sweet), Sapindaceae. Int. J. Pharm. Sci. Res. 2015, 6 (2), 348–351.
Omotayo, I. A.; John, A. A.; Gbenga, O. O.; Misbaudeen, A.-H.; Felix, L. D.; Kolawole, O. A.; Banjo, S. In-silico assessment via molecular docking and ADMET profile of Botanical drugs (bergamottin and casticin) against trial drugs for Lassa virus. Int. J. Pharm. Sci. Res. 2022, 13 (9), 3494–3518. https://doi.org/10.13040/IJPSR.0975-8232.13(9).3494-18
Oyewole, R. O.; Oyebamiji, A. K.; Semire, B. Theoretical calculations of molecular descriptors for anticancer activities of 1,2,3-triazole-pyrimidine derivatives against gastric cancer cell (MGC-803): DFT, QSAR and docking approaches. Heliyon. 2020, 6 (5), e03926. https://doi.org/10.1016/j.heliyon.2020.e03926
Pamplona-Roger, G. D. Encylopedia of medicinal plants; Education and Health Library, 2004.
Rambo, A.-M.; Soares, K. D.; Danielli, J. L.; Lana, D. F. D.; Bordignon, L. A. S.; Fuentefria, M. A.; Apel, M. A. Biological Activities of Essential Oils from Six Genotypes of Four ocotea Species. Braz. J. Pharm. Sci. 2022, 58, e181097. https://doi.org/10.1590/s2175-97902022e181097
Rates, S. M. Plants as source of drugs. Toxicon. 2001, 39 (5), 603–613. https://doi.org/10.1016/S0041-0101(00)00154-9
Seeliger, D.; Groot, B. L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24 (5), 417–422. https://doi.org/10.1007/s10822-010-9352-6
Sohail, T.; Ferheen, S.; Imran, H.; Yaqueen Z.; Rehma, A.; Khan, R. A. Phytochemical and antibacterial screening of different fractions of root part Ipomea Turpethum. Bangladesh J. Med. Sci. 2018, 17 (1), 93–97. https://doi.org/10.3329/bjms.v17i1.35288
Tanchuk, V. Y.; Tanin, V. O.; Vovk, A. I.; Poda, G. A. New, Improved Hybrid Scoring Function for Molecular Docking and Scoring Based on AutoDock and AutoDock Vina. Chem. Biol. Drug Des. 2015, 87 (4), 618–625. https://doi.org/10.1111/cbdd.12697
United Nations Educational, Scientific and Cultural Organization (UNESCO). Traditional Knowledge into the twenty-first century: Nature and Resources; UNESCO, 1994.
World Health Organization (WHO). World Malaria Report; WHO, 2008.
Zeleke, D.; Eswaramoorthy, R.; Belay, Z.; Melaku, Y. Synthesis and Antibacterial, Antioxidant, and Molecular Docking Analysis of Some Novel Quinoline Derivatives. J. Chem. 2020, 2020, 1324096. https://doi.org/10.1155/2020/1324096
Zubair, M.; Bibi, Z.; Rizwan, K.; Rasool, N.; Zahoor F.A.; Riaz, M. In-vitro antimicrobial and Haemolytic Studies of Bambusa nrundinaceae leaves. J. App. Pharm. Sci. 2013, 3 (4), 111–115. https://doi.org/10.7324/JAPS.2013.3420

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Eclética Química