Abstract
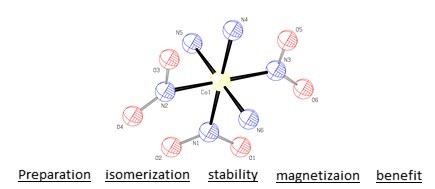
[Co(NH3)3(NO2)3] is an octahedral complex compound that can have several isomers. The complex compound has magnetic properties. Its stability has been explained. It can be easily synthesized and is known as a bioinorganic synthesis reagent, oxidant compound and base hydrolysis.
References
Abd-Ulgadir, K.; El-Kamali, H. Antimicrobial activity of Acacia nilotica ssp. nilotica against some causative agents of urogenital infections. Annu Res Rev Biol. 2017, 19 (5), 1–14. https://doi.org/10.9734/ARRB/2017/36026
Adzamli, I. K.; Kim, H. O.; Sykes, A. G. Neutral complexes as oxidants for the reduced form of parsley (Petroselinum crispum) [2Fe--2S] ferredoxin. Evidence for partial blocking by redox-inactive Cr(III) complexes. Biochem. J. 1982, 203 (3), 669–673. https://doi.org/10.1042/bj2030669
Alhafez, A.; Aytar, E.; Kilic, A. Enhancing catalytic strategy for cyclic carbonates synthesized from CO2 and epoxides by using cobaloxime-based double complex salts as catalysts. J. CO2 Util. 2022, 63 (8), 102129. https://doi.org/10.1016/j.jcou.2022.102129
Bernal, I.; Cetrullo, J.; Somoza, F.; Ricci, J. S.; Lewis, R.; Massoud, S. S. The phenomenon of conglomerate crystallization. Part 42: The crystallization mode and conformational and configurational behavior of mer-trinitro cobalt triamines: mer-[Co(NH3)3(NO2)3] (I) and mer- [Co(dpt)(NO2)3] (II). J. Coord. Chem. 1996, 38 (1–2), 41–53. https://doi.org/10.1080/00958979608022689
Constable, E. C.; Housecroft, C. E. Coordination chemistry: the scientific legacy of Alfred Werner. Chem. Soc. Rev. 2013, 42 (4), 1429–1439. https://doi.org/10.1039/c2cs35428d
Deblitz, R.; Hrib, C. G.; Blaurock, S.; Jones, P. G.; Plenikowski, G.; Edelmann, F. T. Explosive Werner-type cobalt(III) complexes. Inorg. Chem. Front. 2014, 1 (8), 621–640. https://doi.org/10.1039/C4QI00094C
Effendy. Perspektif Baru Kimia Koordinasi; Indonesian Academic Publishing, 2013.
Eivazihollagh, A.; Svanedal, I.; Edlund, H.; Norgren, M. On chelating surfactants: Molecular perspectives and application prospects. J. Mol. Liq. 2019, 278, 688–705. https://doi.org/10.1016/j.molliq.2019.01.076
Ernst, K.-H.; Wild, F. R. W. P.; Blacque, O.; Berke, H. Alfred Werners coordination chemistry: New insights from old samples. Angew. Chemie Int. Ed. 2011, 50 (46), 10780–10787. https://doi.org/10.1002/anie.201104477
Feng, Y.; Yang, H.; Wang, X.; Hu, C.; Jing, H.; Cheng, J. Role of transition metals in catalyst designs for oxygen evolution reaction: A comprehensive review. Int. J. Hydrogen Energy. 2022, 47 (41), 17946–17970. https://doi.org/10.1016/j.ijhydene.2022.03.270
Habiddin, H.; Hartanto, D. Synthesis, characterization and application of complex compounds: Review of studies among Indonesian scholars. AIP Conf. Proc. 2023, 2569, 70001. https://doi.org/10.1063/5.0112075
Kılıç, A.; Durgun, M.; Erdoğan, A. The Novel bis-dioxime-based and boronic acid-capped groups containing Fe(II) and Co(II) complexes: Synthesis, characterization and spectroscopy. J. Turkish Chem. Soc. Sect. A Chem. 2017, 4, 11–22. https://doi.org/10.18596/jotcsa.318141
Kilic, A.; Fırat, H.; Aytar, E.; Durgun, M.; Baytak, A. K.; Aslanoglu, M.; Ulusoy, M. Dicobaloxime/organodicobaloximes bridged by different axial groups: synthesis, characterization, spectroscopy, and catalysis. Chem. Pap. 2017, 71, 1705–1720. https://doi.org/10.1007/s11696-017-0165-0
Kilic, A.; Keles, A.; Aytar, E.; Durgun, M.; Ulusoy, M. Synthesis of the multinuclear cobaloxime complexes via click chemistry as catalysts for the formation of cyclic carbonates from carbon dioxide and epoxides. J. Chem. Sci. 2015, 127, 1665–1674. https://doi.org/10.1007/s12039-015-0932-9
Laing, M.; Baines, S.; Sommerville, P. Crystal structure of trinitrotriamminecobalt(III). Redetermination. Inorg. Chem. 1971, 10 (5), 1057–1061. https://doi.org/10.1021/ic50099a040
Nguyen, D. T.; Cavazos, R. J.; Harris, A. N.; Petros, R. A. Werner complexes viewed anew: Utilizing cobalt coordination chemistry for ‘traceless’ stimuli-responsive bioconjugation involving therapeutic nanoparticles, protein PEGylation, and drug-(bio)polymer conjugates. Comments Inorg. Chem. 2014, 34 (3–4), 59–77. https://doi.org/10.1080/02603594.2014.940103
Nuber, B.; Siebert, H.; Weidenhammer, K.; Weiss, J.; Ziegler, M. L. The crystal structure of fac-triamminetrinitrocobalt(III), fac-[Co(NH3)3(NO2)3. Acta Crystallogr. Sect. B 1979, B35, 1020–1023. https://doi.org/10.1107/S0567740879005483
Palmer, B. J.; Hill, R. H. Solid state photochemistry of fac-Co(NH3)3(NO2)3 and mer-Co(NH3)3(N3)3 as thin films on Si(111) surfaces. J. Photochem. Photobiol. A Chem. 1993, 72, 243–249. https://doi.org/10.1016/1010-6030(93)80020-A
Renfrew, A. K.; O’Neill, E. S.; Hambley, T. W.; New, E. J. Harnessing the properties of cobalt coordination complexes for biological application. Coord. Chem. Rev. 2018, 375, 221–233. https://doi.org/10.1016/j.ccr.2017.11.027
Singh, S.; Shanker, R. Base Hydrolysis of [Co(NO2)3(NH3)3]0. Inorg. Chem. 1989, 28 (14), 2695–2696. https://doi.org/10.1021/ic00313a001
Tanito, Y.; Saito, Y.; Kuroya, H. The crystal structure of trinitrotriammine-cobalt (III), [Co(NH3)3(NO2)3]. Bull. Chem. Soc. Jpn. 1952, 25 (3), 188–191. https://doi.org/10.1246/bcsj.25.188
Bąk, J. M.; Effendy; Grabowsky, S.; Lindoy, L. F.; Price, J. R.; Skelton, B. W.; White, A. H. True and quasi-isomorphism in tetrakis(acetonitrile)coinage metal(I) salts. Cryst. Eng. Comm. 2013, 15, 1125–1138. https://doi.org/10.1039/c2ce26824h
Verma, C.; Quraishi, M. A.; Rhee, K. Y. Natural ligands: Promising ecofriendly alternatives for corrosion protection and plethora of many prospects. Process Saf. Environ. Prot. 2022, 162, 253–290. https://doi.org/10.1016/j.psep.2022.04.014

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Eclética Química