Abstract
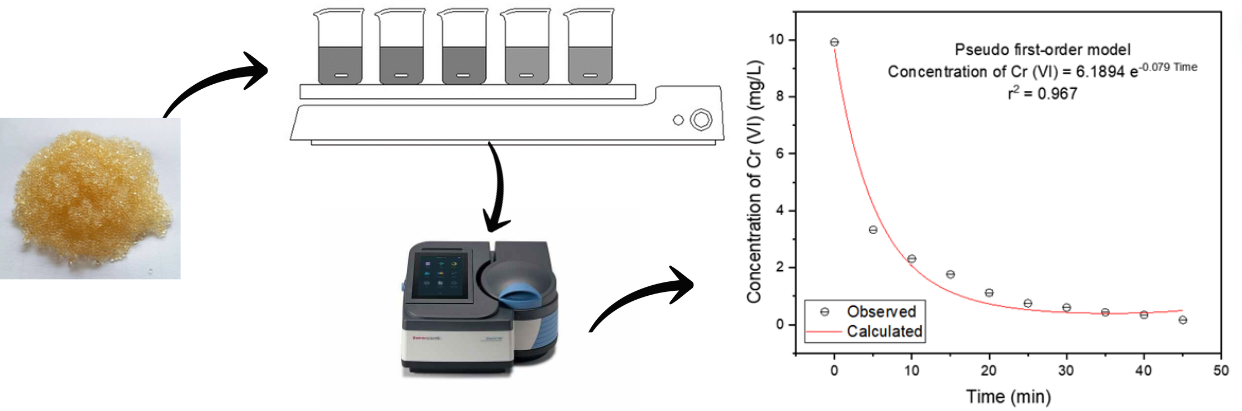
This research aimed to identify optimal studied variables for chromium (VI) removal using four resins (IRA 96, IRA 400, DOWEX 1x8, and LEWATIT). A 1,5-diphenylcarbazide method was used for the quantification of chromium (VI). A factorial design with triple replication at the center point was used to evaluate pH, resin dose (g/100 mL), and initial chromium (VI) concentration. The optimal values for the four resins were a pH of 3, a resin concentration of 0.15 g/100 mL of solution, and an initial concentration of 10 mg/L of chromium. Then, an ANOVA study was done to compare the resins results using a p-value <0.05. The DOWEX resin presented the highest removal percentage (98.39%) for a reaction period of 45 minutes, with an exponential model that fits a pseudo-first-order kinetics with a coefficient of determination equal to 0.967.
References
Bajpai, S.; Dey, A.; Jha, M. K.; Gupta, S. K.; Gupta, A. Removal of hazardous hexavalent chromium from aqueous solution using divinylbenzene copolymer resin. Int. J. Environ. Sci. Technol. 2012, 9 (4), 683–690. https://doi.org/10.1007/s13762-012-0099-6
Bhatti, A. A.; Memon, S.; Memon, N.; Bhatti, A. A.; Solangi, I. B. Evaluation of chromium(VI) sorption efficiency of modified Amberlite XAD-4 resin. Arab. J. Chem. 2017, 10 (Suppl. 1), S1111–S1118. https://doi.org/10.1016/j.arabjc.2013.01.020
Coşkun, R.; Er, E.; Delibaş, A. Synthesis of novel resin containing carbamothiolylimidamide group and application for Cr(VI) removal. Polym. Bull. 2018, 75 (3), 963–983. https://doi.org/10.1007/s00289-017-2068-1
Costa, A. W. M. C. Produção de biossorvente magnetizado à base de biopolímeros do tipo polissacarídeo, para remoção de cromo (VI) de efluentes industriais. Tese (Doutorado em Biotecnologia), Universidade Federal de Sergipe, São Cristóvão, SE, 2017.
Gaikwad, M. S.; Balomajumder, C. Simultaneous rejection of fluoride and Cr(VI) from synthetic fluoride-Cr(VI) binary water system by polyamide flat sheet reverse osmosis membrane and prediction of membrane performance by CFSK and CFSD models. J. Mol. Liq. 2017, 234, 194–200. https://doi.org/10.1016/j.molliq.2017.03.073
Gorman, C.; Seidel, C.; Henrie, T.; Huang, L.; Thompson, R. Pilot Testing Strong Base Anion Exchange for CrVI Removal. Journal AWWA, 2016, 108 (4), E240–E246. https://doi.org/10.5942/jawwa.2016.108.0028
Hashem, A.; Momen, A.; Hasan, M.; Nur-A-Tomal, S.; Sheikh, H. R. Chromium removal from tannery wastewater using Syzygium cumini bark adsorbent. Int. J. Environ. Sci. Technol. 2018, 16, 1395–1404. https://doi.org/10.1007/s13762-018-1714-y
Hu, S.; Li, D.; Huang, C.; Sun, D.; Yuan, X. A continuous electrocoagulation system with pH auto-adjusting by endogenous products to treat Cr(VI)-contaminated soil flushing solution. Sep. Purif. Technol. 2017, 189, 213–219. https://doi.org/10.1016/j.seppur.2017.07.081
Kahraman, H. T.; Pehlivan, E. Evaluation of anion-exchange resins on the removal of Cr(VI) polluted water: Batch ion-exchange modeling. Arab. J. Geosci. 2019, 12, 532. https://doi.org/10.1007/s12517-019-4677-5
Korak, J. A.; Huggins, R.; Arias-Paic, M. Regeneration of pilot-scale ion exchange columns for hexavalent chromium removal. Water Res. 2017, 118, 141–151. https://doi.org/10.1016/j.watres.2017.03.018
Kusku, O.; Rivas, B. L.; Urbano, B. F.; Arda, M.; Kabay, N.; Bryjak, M. A comparative study of removal of Cr(VI) by ion exchange resins bearing quaternary ammonium groups. J. Chem. Technol. Biotechnol. 2014, 89 (6), 851–857. https://doi.org/10.1002/jctb.4320
Li, X.; Shi, S.; Cao, H.; Li, Y.; Xu, D. Comparative Study of Chromium(VI) Removal from Simulated Industrial Wastewater with Ion Exchange Resins. Russ. J. Phys. Chem. A. 2018, 92, 1229–1236. https://doi.org/10.1134/S0036024418060237
Liu, X.; Li, Y.; Wang, C.; Ji, M. Cr(VI) removal by a new type of anion exchange resin DEX-Cr: Adsorption affecting factors, isotherms, kinetics, and desorption regeneration. Environ. Prog. Sustain. Energy. 2015, 34 (2), 387–393. https://doi.org/10.1002/ep.11998
Ok, Y. S.; Jeon, C. Selective adsorption of the gold–cyanide complex from waste rinse water using Dowex 21K XLT resin. J. Ind. Eng. Chem. 2014, 20 (4), 1308–1312. https://doi.org/10.1016/j.jiec.2013.07.010
Patel, P. K.; Nagireddi, S.; Uppaluri, R. V. S.; Pandey, L. M. Batch adsorption characteristics of Dowex Marathon MSA commercial resin for Au(III) removal from synthetic electroless plating solutions. Mater. Today. Proc. 2022, 68 (4), 824–829. https://doi.org/10.1016/j.matpr.2022.06.258
Pflaum, R. T.; Howick, L. C. The Chromium-Diphenylcarbazide Reaction. J. Am. Chem. Soc. 1956, 78, 4862–4866. https://doi.org/10.1021/ja01600a014
Polowczyk, I.; Urbano, B. F.; Rivas, B. L.; Bryjak, M.; Kabay, N. Equilibrium and kinetic study of chromium sorption on resins with quaternary ammonium and N-methyl-d-glucamine groups. Chem. Eng. J. 2016, 284, 395–404. https://doi.org/10.1016/j.cej.2015.09.018
Sadyrbaeva, T. Z. Removal of chromium (VI) from aqueous solutions using a novel hybrid liquid membrane—Electrodialysis process. Chem. Eng. Process.: Process Intensif. 2016, 99, 183–191. https://doi.org/10.1016/j.cep.2015.07.011
Tümer, A. E.; Edebali, S. Artificial Neural Network Approach for Modeling of Cr(VI) Adsorption from Waste Water by Lewatit MP64 and Dowex 1×8. 2019 IEEE 7th Palestinian International Conference on Electrical and Computer Engineering (PICECE), Gaza, Palestine, 2019. https://doi.org/10.1109/PICECE.2019.8747199
Wang, C.-C.; Du, X.-D.; Li, J.; Guo, X.-X.; Wang, P.; Zhang, J. Photocatalytic Cr(VI) reduction in metal-organic frameworks: A mini-review. Appl. Catal. B: Environ. 2016, 193, 198–216. https://doi.org/10.1016/j.apcatb.2016.04.030
Xie, B.; Shan, C.; Xu, Z.; Li, X.; Zhang, X.; Chen, J.; Pan, B. One-step removal of Cr(VI) at alkaline pH by UV/sulfite process: Reduction to Cr(III) and in situ Cr(III) precipitation. Chem. Eng. J. 2017, 308, 791–797. https://doi.org/10.1016/j.cej.2016.09.123
Xing, X.; Alharbi, N. S.; Ren, X.; Chen, C. A comprehensive review on emerging natural and tailored materials for chromium-contaminated water treatment and environmental remediation. J. Environ. Chem. Eng. 2022, 10 (2), 107325. https://doi.org/10.1016/j.jece.2022.107325

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Eclética Química