Abstract
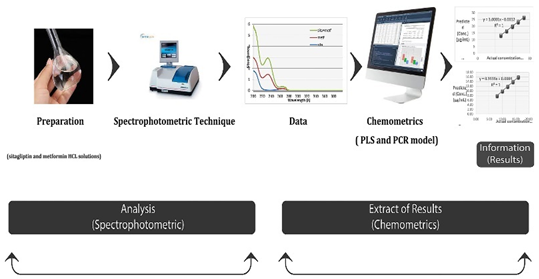
A new, quick, easy, affordable and eco-friendly simultaneous spectrophotometric method for determining a combined sitagliptin and metformin hydrochloride in pharmaceutical formulations was developed and validated using two chemometrics technique. These two methods are the partial least square (PLS) and principal component regression (PCR). They do not need to do a sample preparation or separation before analysis. Various drug concentrations and instrumental spectra of 25 mixed solutions of a combination of sitagliptin and metformin hydrochloride were used for model construction in the range of 200–270 nm. The R2 values of 0.9994 and 0.9996 assigned for the PLS of the sitagliptin and metformin hydrochloride and that of 0.9987 and 0.9996 for the PCR of the sitagliptin and metformin hydrochloride, respectively. It is noteworthy that these two models were successfully and effectively used with the commercial pharmaceutical formulations. Finally, the statistical comparison revealed no significant differences with the results of the HPLC reference method. The proposed method is dependable to be adopted as an alternative analytical method in the pharmaceutical industry’s quality control.
References
Adsul, S.; Bidkar, J. S.; Harer, S.; Dama, G. Y. RP-HPLC method development and validation for simultaneous estimation for metformin and sitagliptin in bulk and tablet formulation. Int. J. Chem. Tech. Res. 2018, 11 (11), 428–435. https://doi.org/10.20902/IJCTR.2018.111149
Aminu, N.; Chan, S.-Y.; Khan, N. H.; Farhan, A. B.; Umar, M. N.; Toh, S.-M. A simple stability-indicating HPLC method for simultaneous analysis of paracetamol and caffeine and its application to determinations in fixed-dose combination tablet dosage form. Acta Chromatogr. 2019, 31 (2), 85–91. https://doi.org/10.1556/1326.2018.00354
Ashour, A.; Hegazy, M. A.; Abdel-Kawy, M.; ElZeiny, M. B. Simultaneous spectrophotometric determination of overlapping spectra of paracetamol and caffeine in laboratory prepared mixtures and pharmaceutical preparations using continuous wavelet and derivative transform. J. Saudi Chem. Soc. 2015, 19 (2), 186–192 https://doi.org/10.1016/j.jscs.2012.02.004
Attia, K. A.-S. M.; Abdel-Aziz, O.; Magdy, N.; Mohamed, G. F. Development and validation of different chemometric-assisted spectrophotometric methods for determination of cefoxitin-sodium in presence of its alkali-induced degradation product. Future J. Pharm. Sci. 2018, 4 (2), 241–247 https://doi.org/10.1016/j.fjps.2018.08.002
Belal, F.; Ibrahim, F.; Sheribah, Z.; Alaa, H. New spectrophotometric/chemometric assisted methods for the simultaneous determination of imatinib, gemifloxacin, nalbuphine and naproxen in pharmaceutical formulations and human urine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 198, 51–60. https://doi.org/10.1016/j.saa.2018.02.048
British Pharmacopoeia Commission. Medicines and Healthcare products Regulatory Agency (MHRA). British Pharmacopoeia Commission. 2020, 3 (6), 1844.
Darbandi, A.; Sohrabi, M. R.; Bahmaei, M. Development of a chemometric-assisted spectrophotometric method for quantitative simultaneous determination of Amlodipine and Valsartan in commercial tablet. Optik. 2020, 218, 165110. https://doi.org/10.1016/j.ijleo.2020.165110
Elfatatry, H. M.; Mabrouk, M. M.; Hammad, S. F.; Mansour, F. R.; Kamal, A. H.; Alahmad, S. Development and validation of chemometric-assisted spectrophotometric methods for simultaneous determination of phenylephrine hydrochloride and ketorolac tromethamine in binary combinations. J. AOAC Inter. 2016, 99 (5), 1247–1251. https://doi.org/10.5740/jaoacint.16-0106
Gandhi, S. V.; Waghmare, A. D.; Nandwani, Y. S.; Mutha, A. S. Chemometrics - Assisted UV spectrophotometric method for determination of ciprofloxacin and ornidazole in pharmaceutical formulation. ARC Journal of Pharmaceutical Sciences. 2017, 3 (1), 19–25. https://doi.org/10.20431/2455-1538.0301005
Gholse, Y. N.; Chaple, D. R.; Kasliwal, R. H. Development and validation of novel analytical simultaneous estimation based UV spectrophotometric method for doxycycline and levofloxacin determination. Biointerface Res. Appl. Chem. 2021, 12 (4), 5458–5478. https://doi.org/10.33263/BRIAC124.54585478
Glavanović, S.; Glavanović, M.; Tomišić, V. Simultaneous quantitative determination of paracetamol and tramadol in tablet formulation using UV spectrophotometry and chemometric methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 157, 258–264. https://doi.org/10.1016/j.saa.2015.12.020
Himabindu, T.; Narmadha, S.; Sireesha, D.; Vasudha, B. Development and validation of spectrophotometric method for the simultaneous estimation of metformin hydrochloride and sitagliptinin tablet dosage form. World J. Pharm. Res. 2016, 5 (7), 1011–1018.
Krishnan, B.; Mishra, K. Quality by design based development and validation of RP-HPLC method for simultaneous estimation of sitagliptin and metformin in bulk and pharmaceutical dosage forms. Int. J. Pharm. Investig. 2020, 10 (4), 512–518. https://doi.org/10.5530/ijpi.2020.4.89
Kumar, V. P.; Kavitha, M.; Patro, S.; Bhavya, C.; Bag, A. K. Development and validation of new analytical method for the simultaneous estimation of metformin and sitagliptin in bulk and dosage form by RP-HPLC. World J. Pharm. Res. 2017, 6 (3), 1691–1700.
Lotfy, H. M.; Mohamed, D.; Mowaka, S. A comparative study of smart spectrophotometric methods for simultaneous determination of sitagliptinand metformin hydrochloride in their binary mixture. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 149, 441–451. https://doi.org/10.1016/j.saa.2015.04.076
Manouchehri, F.; Izadmanesh, Y.; Aghaee, E.; Ghasemi, J. B. Experimental, computational and chemometrics studies of BSA-vitamin B6 interaction by UV–Vis, FT-IR, fluorescence spectroscopy, molecular dynamics simulation and hard-soft modeling methods. Bioorg. Chem. 2016, 68, 124–136. https://doi.org/10.1016/j.bioorg.2016.07.014
Mohammed, O. J.; Hamzah, M. J.; Saeed, A. M. RP–HPLC method validation for simultaneous estimation of paracetamol and caffeine in formulating pharmaceutical form. Res. J. Pharm. Technol. 2021, 14 (9), 4743–4748. https://doi.org/10.52711/0974-360X.2021.00825
Moroni, A. B.; Vega, D. R.; Kaufman, T. S.; Calvo, N. L. Form quantitation in desmotropic mixtures of albendazole bulk drug by chemometrics-assisted analysis of vibrational spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120354. https://doi.org/10.1016/j.saa.2021.120354
Moussa, B. A.; Mahrouse, M. A.; Fawzy, M. G. Smart spectrophotometric methods for the simultaneous determination of newly co-formulated hypoglycemic drugs in binary mixtures. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 257, 119763 https://doi.org/10.1016/j.saa.2021.119763
Muntean, D. M.; Alecu, C.; Tomuta, I. Simultaneous quantification of paracetamol and caffeine in powder blends for tableting by NIR-chemometry. J. Spectroscopy. 2017, 2017, 7160675. https://doi.org/10.1155/2017/7160675
Muntean, D.; Porfire, A.; Alceu, C.; Iurian, S.; Casian, T.; Gavan, A.; Tomuta, I. A non-destructive NIR spectroscopic method combined with chemometry for simultaneous assay of paracetamol and caffeine in tablets. Ro. J. Pharm. Pract. 2021, 14 (2), 68-75. https://doi.org/10.37897/RJPhP.2021.2.2
Ortega-Barrales, P.; Padilla-Weigand, R.; Molina-Díaz, A. Simultaneous determination of paracetamol and caffeine by flow injection–solid phase spectrometry using C18 silica gel as a sensing support. Anal. Sci. 2002, 18 (11), 1241–1246. https://doi.org/10.2116/analsci.18.1241
Patel, K. R.; Prajapati, L. M.; Joshi, A. K.; Kharodiya, M. L.; Patel, J. R. Application of chemometrics in simultaneous spectrophotometric quantification of etophylline and theophylline: The drugs with same chromophore. Iranian Journal of Pharmaceutical Sciences 2013a, 9 (3), 17–28.
Patel, M. N.; Alvi, S. N.; Savalia, M. D.; Kathiria, P. B.; Patel, B. A.; Parmar, S. J. Development and validation of first order derivative spectrophotometric method for simultaneous estimation of paracetamol and caffeine in tablet dosage form. Inventi Rapid: Pharm Analysis & Quality Assurance. 2013b, 2013 (2), 1–5.
Patel, R.; Mashru, R. Development and validation of chemometric assisted methods and stability indicating RP-HPLC method for simultaneous estimation of rasagiline mesylate and pramipexole in synthetic mixture. Acta Scientific Pharmaceutical Sciences. 2019, 3 (8), 154–168. https://doi.org/10.31080/ASPS.2019.03.0359
Phechkrajang, C. M.; Siriratawan, W.; Narapanich, K.; Thanomchat, K.; Kantanawat, P.; Srikajhondei, W.; Khajornvanitchot, V.; Sakchaisri, K. Development and validation of chemometrics-assisted spectrophotometric method for determination of clotrimazole in the presence of betamethasone valerate. Mahidol University J. Pharm. Sci. 2015, 42 (2), 1–7.
Putri, D. C. A.; Gani, M. R.; Octa, F. D. Chemometrics-assisted UV spectrophotometric method for simultaneous determination of paracetamol and tramadol in divided powder dosage form. Int. J. Pharm. Res. 2021, 13 (1), 1901–1907. https://doi.org/10.31838/ijpr/2021.13.01.075
Rahman, A.; Sravani, G. J.; Srividya, K.; Priyadharshni, A. D. R.; Narmada, A.; Sahithi, K.; Sai, T. K.; Padmavathi, Y. Development and validation of chemometric assisted FTIR spectroscopic method for simultaneous estimation of valsartan and hydrochlorothiazide in pure and pharmaceutical dosage forms. J. Young Pharm. 2020, 12 (2s), s51-s55. https://doi.org/10.5530/jyp.2020.12s.46
Salem, Y. A.; Hammouda, M. E. A.; El-Enin, M. A. A.; El-Ashry, S. M. Application of derivative emission fluorescence spectroscopy for determination of ibuprofen and phenylephrine simultaneously in tablets and biological fluids. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 387–397. https://doi.org/10.1016/j.saa.2018.11.054
Sebaiy, M. M.; El-Adl, S. M.; Mattar, A. A. Different techniques for overlapped UV spectra resolution of some co-administered drugs with paracetamol in their combined pharmaceutical dosage forms. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117429. https://doi.org/10.1016/j.saa.2019.117429
Sebaiy, M.; Mattar, A. A.; El-Adl, S. M. UV-chemometric method development for resolving the overlapped spectra of aspirin, caffeine and orphenadrine citrate in their ternary pharmaceutical dosage form. Research Square. Preprint; 2022. https://doi.org/10.21203/rs.3.rs-1262160/v1
Shah, U. H.; Jasani, A. H. Chemometric assisted spectrophotometric methods for simultaneous determination of paracetamol and tolperisone hydrochloride in pharmaceutical dosage form. Eurasian J. Anal. Chem. 2017, 12 (3), 211–222.
Shinde, M. A.; Divya, O. Simultaneous quantitative analysis of a three-drug combination using synchronous fluorescence spectroscopy and chemometrics. Current Science. 2015, 108 (7), 1348–1354.
Silva, W. C.; Pereira, P. F.; Marra, M. C.; Gimenes, D. T.; Cunha, R. R.; Silva, R. A.; Munoz, R. A.; Richter, E. M. A simple strategy for simultaneous determination of paracetamol and caffeine using flow injection analysis with multiple pulse amperometric detection. Electroanalysis. 2011, 23 (12), 2764–2770. https://doi.org/10.1002/elan.201100512
Singh, V. D.; Singh, V. K. Chemo-metric assisted UV-spectrophotometric methods for simultaneous estimation of Darunavir ethanolate and Cobicistat in binary mixture and their tablet formulation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119383. https://doi.org/10.1016/j.saa.2020.119383
Sun, X.; Li, H.; Yi, Y.; Hua, H.; Guan, Y.; Chen, C. Rapid detection and quantification of adulteration in Chinese hawthorn fruits powder by near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119346. https://doi.org/10.1016/j.saa.2020.119346
Swamy, G. K.; Surekha, M. L.; Krishna, M. M. Development and validation of RP-HPLC method for simultaneous estimation of metformin and sitagliptin in bulk and tablet dosage forms. Journal of Pharmaceutical and Medicinal Chemistry. 2020, 6 (1), 15–20.
Tsvetkova, B.; Kostova, B.; Pencheva, I.; Zlatkov, A.; Rachev, D.; Peikov, P. Validated LC method for simultaneous analysis of paracetamol and caffeine in model tablet formulation. Int. J. Pharm. Pharm. Sci. 2012, 4 (Suppl. 4), 680–684.
Uddin, M.; Mondol, A.; Karim, M.; Jahan, R.; Rana, A. Chemometrics assisted spectrophotometric method for simultaneous determination of paracetamol and caffeine in pharmaceutical formulations. Bangladesh J. Sci. Ind. Res. 2019, 54 (3), 215–222. https://doi.org/10.3329/bjsir.v54i3.42673
United States Pharmacopeia and the National Formulary (USP 43 - NF 38). The United States Pharmacopeial Convention; 2020. https://www.uspnf.com/notices/usp-nf-final-print-edition (accessed 2022-06-09).
Vichare, V.; Mujgond, P.; Tambe, V.; Dhole, S. N. Simultaneous Spectrophotometric Determination of Paracetamol and Caffeine in Tablet Formulation. Int. J. PharmTech Res. 2010, 2 (4), 2512–2516.
Vu Dang, H.; Thu, H. T. T.; Ha, L. D. T.; Mai, H. N. RP-HPLC and UV Spectrophotometric Analysis of Paracetamol, Ibuprofen, and Caffeine in Solid Pharmaceutical Dosage Forms by Derivative, Fourier, and Wavelet Transforms: A Comparison Study. J. Anal Methods Chem. 2020, 2020, 8107571. https://doi.org/10.1155/2020/8107571
Walash, M. I.; Belal, F. F.; El-Enany, N. M.; El-Maghrabey, M. H. Synchronous fluorescence spectrofluorimetric method for the simultaneous determination of metoprolol and felodipine in combined pharmaceutical preparation. Chem. Central J. 2011, 5, 70. https://doi.org/10.1186/1752-153X-5-70
Zhu, L.; Wu, H.-L.; Xie, L.-X.; Fang, H.; Xiang, S.-X.; Hu, Y.; Liu, Z.; Wang, T.; Yu, R.-Q. A chemometrics-assisted excitation–emission matrix fluorescence method for simultaneous determination of arbutin and hydroquinone in cosmetic products. Analytical Methods. 2016, 8 (24), 4941–4948. https://doi.org/10.1039/C6AY00821F

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Eclética Química