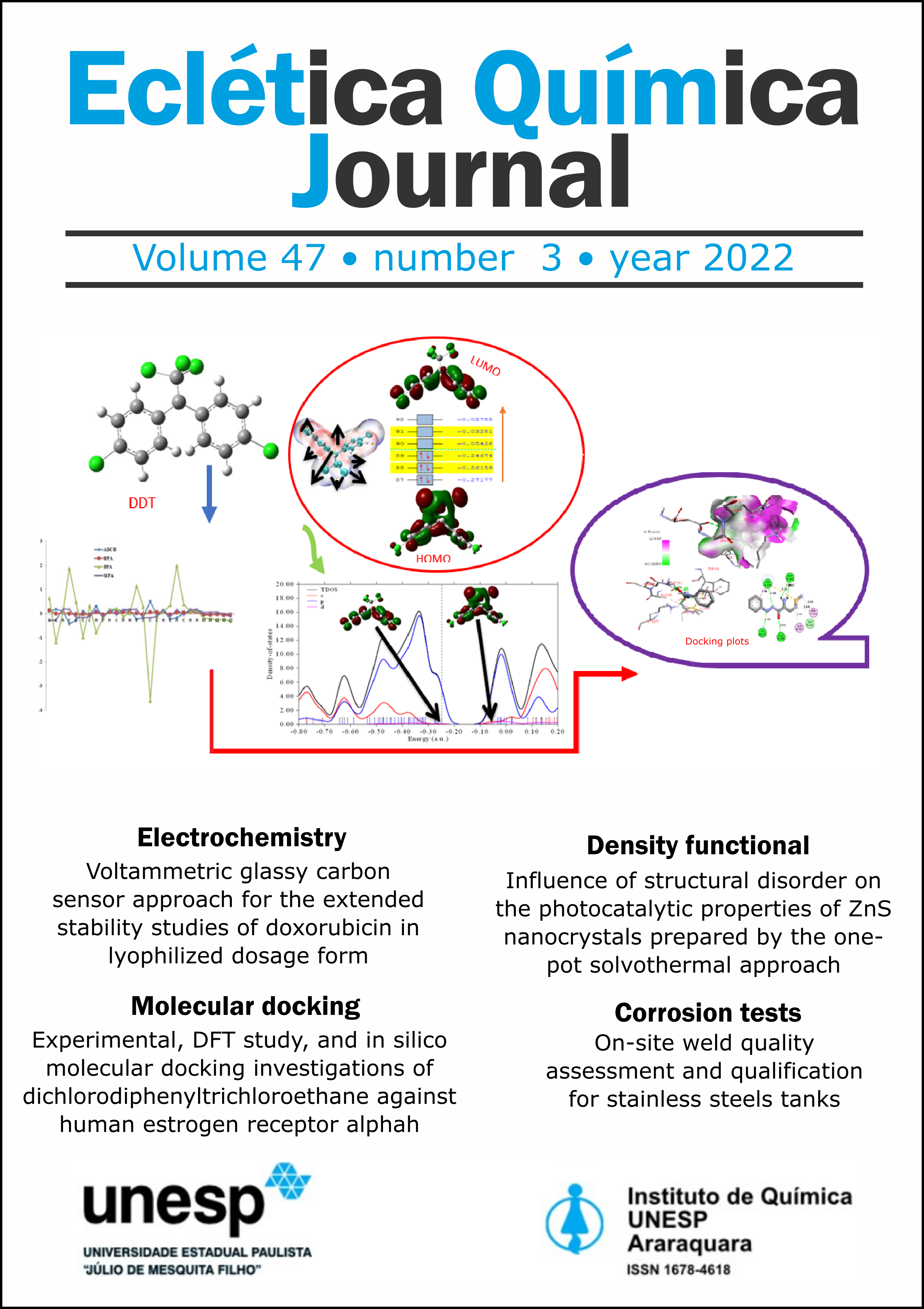
Abstract
Advanced computational tools allowed to study a pure commercial sample of dichlorodiphenyltrichloroethane (DDT) prepared in liquid phase in KBr pellets and characterized using FT-IR and GC-MS followed by the application of DDT for molecular docking against human estrogen receptor alpha. The compound was modelled using GaussView software. Using Veda 04 program, the theoretical vibrational energy distributions and experimental vibrational frequencies were compared. Interestingly, C1 and C2 possess the highest atomic charge density distribution (ACDD) of -0.284e and -0.283e while C21 and C11 have lowest ACDD of -0.064e and -0.063e in a relative manner, since the deactivating power of chlorine atoms decreases charge densities of the bonded carbon. The highest intramolecular interacting perturbation energy is 1121.92 kJ mol–1 occurs between π*C19–C21 donor orbital and π*C14–C16 acceptor orbital while the least intramolecular interaction occurs in the lone pair of LPC26 and the sigma nonbonding (𝜎C1–Cl24) NBO orbitals with E(2) of 32.21 kJ mol–1. Steric interaction was the only interaction found within the complex after the docking.
References
Agwupuye, J. A.; Louis, H.; Enudi, O. C.; Unimuke, T. O.; Edim, M. M. Theoretical insight into electronic and molecular properties of halogenated (F, Cl, Br) and hetero-atom (N, O, S) doped cyclooctane. Mater. Chem. Phys. 2021a, 275, 125239. https://doi.org/10.1016/j.matchemphys.2021.125239
Agwupuye, J. A.; Louis, H.; Unimuke, T. O.; David, P.; Ubana, E. I.; Moshood, Y. L. Electronic structure investigation of the stability, reactivity, NBO analysis, thermodynamics, and the nature of the interactions in methyl-substituted imidazolium-based ionic liquids. J. Mol. Liq. 2021b, 337, 116458. https://doi.org/10.1016/j.molliq.2021.116458
Agwupuye, J. A.; Neji, P. A.; Louis, H.; Odey, J. O.; Unimuke, T. O.; Bisiong, E. A.; Ntui, T. N. Investigation on electronic structure, vibrational spectra, NBO analysis, and molecular docking studies of aflatoxins and selected emerging mycotoxins against wild-type androgen receptor. Heliyon. 2021c, 7 (7), e07544. https://doi.org/10.1016/j.heliyon.2021.e07544
Armaković, S.; Armaković, S. J.; Šetrajčić, J. P.; Šetrajčić, I. J. Active components of frequently used β-blockers from the aspect of computational study. J. Mol. Model. 2012, 18 (9), 4491–4501. https://doi.org/10.1007/s00894-012-1457-5
Barnes K. What is the Steric Effect in Organic Chemistry? - Definition & Examples. Study.com. 2019. https://study.com/academy/lesson/what-is-the-steric-effect-in-organic-chemistry-definition-examples.html#:~:text=The%20steric%20effect%20is%20when,occupy%20the%20same%20physical%20space (accessed 2022-06-26).
Bassey, V. M.; Apebende, C. G.; Idante, P. S.; Louis, H.; Emori, W.; Cheng, C. R.; Asogwa, F. C. Vibrational characterization and molecular electronic investigations of 2-acetyl-5-methylfuran using FT-IR, FT-Raman, UV–VIS, NMR, and DFT methods. J. Fluoresc. 2022, 32 (3), 1005–1017. https://doi.org/10.1007/s10895-022-02903-8
Bhuvaneswari, R.; Nagarajan, V.; Chandiramouli, R. Sensing studies of DDT and Toxaphene molecules using chemi-resistive β-antimonene nanotubes based on first-principles insights. Chem. Phys. Lett. 2020, 757, 137895. https://doi.org/10.1016/j.cplett.2020.137895
Buah-Kwofie, A.; Humphries, M. S.; Pillay, L. Bioaccumulation and risk assessment of organochlorine pesticides in fish from a global biodiversity hotspot: iSimangaliso Wetland Park, South Africa. Sci. Total Environ. 2018, 621, 273–281. https://doi.org/10.1016/j.scitotenv.2017.11.212
Dennington, R.; Keith, T. A.; Millam, J. M. GaussView 6.0. 16. Semichem Inc.: Shawnee Mission, KS, USA. 2016.
Enudi, O. C.; Louis, H.; Edim, M. M.; Agwupuye, J. A.; Ekpen, F. O.; Bisong, E. A.; Utsu, P. M. Understanding the aqueous chemistry of quinoline and the diazanaphthalenes: insight from DFT study. Heliyon. 2021, 7 (7), e07531. https://doi.org/10.1016/j.heliyon.2021.e07531
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J. ; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R..; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09 (Gaussian, Inc., Wallingford CT, 2009).
Glendening, E. D.; Badenhoop, J. K.; Reed, A. E.; Carpenter, J. E.; Bohmann, J. A.; Morales, C. M.; Karafiloglou, P.; Landis, C. R.; Weinhold, F. NBO 7.0, Theoretical Chemistry Institute, University of Wisconsin, Madison, 2018. https://nbo7.chem.wisc.edu/biblio_css.htm (accessed 2022-06-26).
Hovmöller, S.; Smith, G.; Kennard, C. H. L. Structural Studies of Polychlorinated Hydrocarbons. V. 1, 1, 1, 2-Tetrachloro-2, 2-bis (p-chlorophenyl) ethane and 1, 1, 1-Tribromo-2, 2-bis (p-chlorophenyl) ethane. Acta Cryst. 1978, B34 (10), 3016–3021. https://doi.org/10.1107/S0567740878009942
Humphrey, W.; Dalke, A.; Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 1996, 14 (1), 33–38. https://doi.org/10.1016/0263-7855(96)00018-5
HyperChem, T. HyperChem 8.07, HyperChem Professional Program. Gainesville, Hypercube. 2001.
Iramain, M. A.; Castillo, M. V.; Davies, L.; Manzur, M. E.; Brandan, S. A. Structural and SQMFF study of potent insecticide 4′, 4′-DDT combining the FT-IR and FT-Raman spectra with DFT calculations. J. Mol. Struct. 2020, 1199, 126964. https://doi.org/10.1016/j.molstruc.2019.126964
Isborn, C. M.; Leclercq, A.; Vila, F. D.; Dalton, J. L.; Brédas, L. R.; Eichinger, B. E.; Robinson. B. H. Comparison of static first hyperpolarizabilities calculated with various quantum mechanical methods. J. Phys. Chem. A. 2007, 111 (7) 1319–1327. https://doi.org/10.1021/jp064096g
Khalid, M.; Ali, A.; Adeel, M.; Din, Z. U.; Tahir, M. N.; Rodrigues-Filho, E.; Khan, M. U. Facile preparation, characterization, SC-XRD and DFT/DTDFT study of diversely functionalized unsymmetrical bis-aryl-α, β-unsaturated ketone derivatives. J. Mol. Struct. 2020, 1206, 127755. https://doi.org/10.1016/j.molstruc.2020.127755
Kowenje, C. O.; Osewe, E. T.; Lalah, J. O. Effects of faujasite X and Y zeolites on the 1,1,1- trichloro-2, 2’bis (p-chlorophenyl) ethane (DDT) degradation during water purification. Int. J. Environ. Pollut. 2013, 1 (1), 9–15. https://doi.org/10.11648/j.ijepp.20130101.12
Lee, C.; Yang, W.; Parr, R. G. Development of the colle-salveti correlation energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37 (2), 785–789. https://doi.org/10.1103/PhysRevB.37.785
Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo[18]carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. https://doi.org/10.1016/j.carbon.2020.05.023
Lu, T.; Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33 (5), 580–592. https://doi.org/10.1002/jcc.22885
Lu, T. Multiwfn (a multifunctional wavefunction analyzer), software manual. Beijing Kein Research Center for Natural Sciences; Version 3.4, 2017. https://web.mit.edu/multiwfn_v3.4/Manual_3.4.pdf (accessed 2022-06-26).
Mascarenhas, N. M.; Ghoshal, N. An efficient tool for identifying inhibitors based on 3D-QSAR and docking using feature-shape pharmacophore of biologically active conformation–A case study with CDK2/CyclinA. Eur. J. Med. Chem. 2008, 43 (12), 2807–2818. https://doi.org/10.1016/j.ejmech.2007.10.016
Miao, J.; Liu, A.; Wu, L.; Yu, M.; Wei, W.; Liu, S. Magnetic ferroferric oxide and polydopamine molecularly imprinted polymer nanocomposites based electrochemical impedance sensor for the selective separation and sensitive determination of dichlorodiphenyltrichloroethane (DDT). Anal. Chim. Acta. 2020, 1095, 82–92. https://doi.org/10.1016/j.aca.2019.10.027
Pagadala, N. S.; Syed, K.; Tuszynski, J. Software for molecular docking: a review. Biophys. Rev. 2017, 9 (2), 91–102. https://doi.org/10.1007/s12551-016-0247-1
Prasad, P. N.; Ulrich, D. R. Nonlinear optical and electroactive polymers. Springer Science & Business Media, 2012.
Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Tables of spectral data for structure determination of organic compounds. Springer Science & Business Media, 2013.
Ray, P. C. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem. Rev. 2010, 110 (9), 5332–5365. https://doi.org/10.1021/cr900335q
Saminathan, M.; Jayakumar, M. R.; Chandrasekaran, R.; Raja, R.; George, J.; Alagusundaram, P. Synthesis, spectral, crystal structure, drug‐likeness, in silico and in vitro biological screening of halogen [Cl, Br] substituted N‐Phenylbenzo [g] indazole derivatives as antimicrobial agents. J. Heterocycl. Chem. 2021, 58 (3), 841–863. https://doi.org/10.1002/jhet.4219
Sruthi, S. N.; Shyleshchandran, M. S.; Mathew, S. P.; Ramasamy, E. V. Contamination from organochlorine pesticides (OCPs) in agricultural soils of Kuttanad agroecosystem in India and related potential health risk. Environ. Sci. Pollut. Res. 2017, 24 (1), 969–978. https://doi.org/10.1007/s11356-016-7834-3
Suresh, S.; Gunasekaran, S.; Srinivasan, S. Spectroscopic (FT-IR, FT-Raman, NMR and UV– Visible) and quantum chemical studies of molecular geometry, Frontier molecular orbital, NLONBO and thermodynamic properties of salicylic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 130–141. https://doi.org/10.1016/j.saa.2014.04.174
Tadesse, T. Quantum Mechanical Study on the Effect of Solvent in the Properties of Benzophenone. J. Phys. Chem. Biophys. 2017, 7 (4) 1000259. https://doi.org/10.4172/2161-0398.1000259
Yang, W. H.; Wang, Z. Y.; Liu, H. L.; Yu, H. X. Exploring the binding features of polybrominated diphenyl ethers as estrogen receptor antagonists: docking studies. SAR QSAR Environ. Res. 2010, 21 (3–4), 351–367. https://doi.org/10.1080/10629361003773971
Zhang, Y.-x.; Wang, Y.-h. Nonlinear optical properties of metal nanoparticles: a review. RSC Adv. 2017, 7 (71), 45129–45144. https://doi.org/10.1039/C7RA07551K
Zhang, R.; Li, P.; Zhang, R.; Shi, X.; Li, Y.; Zhang, Q.; Wang, W. Computational study on the detoxifying mechanism of DDT metabolized by cytochrome P450 enzymes. J. Hazard. Mater. 2021, 414, 125457. https://doi.org/10.1016/j.jhazmat.2021.125457

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2022 Eclética Química Journal