Abstract
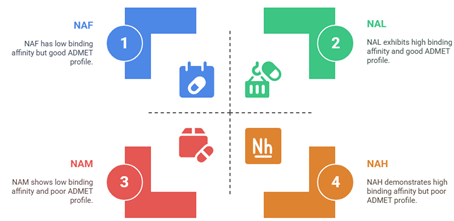
In silico methods were used in this paper to assess the anti-bacterial activity of Sulfamidophosphonate derivatives against Staphylococcus aureus proteins (1XSD and 4WK3) using molecular docking and ADMET analysis. The results showed that binding affinity (ΔG kJ/mol) ranged from –4.1(NAM) to –7.1 kJ/mol (NAL) for 1XSD, and –5.0 (NAE) to –6.7 kJ/mol (NAM) for 4WK3. Therefore, compounds NAH, NAL, NAN, NAI, NAJ, NAK, 5AD and NAM could be more desirable as inhibitors than Penicillin (–6.0 kJ/mol for 1XSD and –5.4 kJ/mol for 4WK3) in the treatment of Staphylococcus aureus; but ADMET profile revealed that compounds NAF, NAI, NAK NAN and 5AC present attractive pharmacokinetic properties. In this study, compounds NAH, NAL, NAI and NAJ exhibited stronger affinities than the standard (penicillin) against BlaI repressor in complex with DNA (PDB ID: 1XSD) suggesting better inhibitory potential than the standard drug.
References
Adegbola, A. E.; Fadahunsi, O. S.; Alausa, A.; Abijo, A. Z.; Balogun, T. A.; Aderibigbe, T. S.; Semire, B.; Adegbola, P. I. Computational prediction of nimbanal as potential antagonist of respiratory syndrome coronavirus. Inform. Med. Unlocked. 2021a, 24, 100617. https://doi.org/10.1016/j.imu.2021.100617
Adegbola, P. I.; Semire, B.; Fadahunsi, O. S.; Adegoke, A. E. Molecular docking and ADMET studies of Allium cepa, Azadirachta indica and Xylopia aethiopica isolates as potential anti-viral drugs for Covid-19. Virus Dis. 2021b, 32, 85-97. https://doi.org/10.1007/s13337-021-00682-7
Adepoju A. J.; Latona D. F.; Oluwafemi Gbenga Olafare O. G.; Oyebamiji A. K.; Misbaudeen Abdul-Hammed M.; Semire B. Molecular docking and pharmacokinetics studies of Curcuma longa (Curcumin) potency against Ebola virus. Ovidius University Annals of Chemistry. 2022, 33 (1), 23–35. https://doi.org/10.2478/auoc-2022-0004
Arora P.; Arora V.; Lamba H.; Wadhwa, D. Importance of Heterocylic Chemistry:A review. International Journal of Pharmaceutical Science. 2012, 3 (9), 2947–2954.
Asibor, Y. E.; Oyebamiji, A. K.; Latona, D. F.; Semire, B. Computational screening of phytochemicals present in some Nigerian medicinal plants against sickle cell disease. Scientific Reports. 2024, 14 (1), 26368. https://doi.org/10.1038/s41598-024-75078-w
Bazine, I.; Bendjedid, S.; Boukhari, A. Potential antibacterial and antifungal activities of novel sulfamidophosphonate derivatives bearing the quinoline or quinolone moiety. Arch Pharm. 2020, 354, e2000291. https://doi.org/10.1002/ardp.202000291
Bazine, I.; Cheraiet, Z.; Bensegueni, R.; Bensouici, C.; Boukhari A. Antioxidant and anticholinesterase activities of novel quinoline-aminophosphonate derivatives. Journal of Heterocyclic Chemistry. 2020, 57 (5), 2139–2149. https://doi.org/10.1002/jhet.3933
Becke, A. Density functional thermochemistry. III. The role of exact exchange. Journal of Physical Chemistry. 1993, 98 (7), 5648–5652. https://doi.org/10.1063/1.464913
Conrad, J.; Paras, N. A.; Vaz, R. J. Model of P-Glycoprotein Ligand Binding and Validation with efflux substrate Matched Pairs. Journal of Medicinal Chemistry. 2024, 67 (7), 5854–5865. https://doi.org/10.1021/acs.jmedchem.4c00139
Daina, A.; Zoete, V. ABOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11 (11), 1117–1221. https://doi.org/10.1002/cmdc.201600182
Daina, A.; Michielin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug likeness and medicinal chemistry friendliness of small molecules. Scientific Reports. 2017, 7, 1−13. https://doi.org/10.1038/srep42717
Darvas, F.; Keseru, G.; Papp, A.; Dorma´n, G.; Urge, L.; Krajcsi, P. In Silico and ex silico ADME Approaches for drug discovery. Topics in Medicinal Chemistry. 2002, 2, 1287–1304. https://doi.org/10.2174/1568026023392841
Farhan, A. K.; Sidra, M.; Sadia, N.; Umar, F.; Asma, Z.; Syed Majid, B.; Abdur, R.; Mohammad, S. M. Sulfonamides as potential bioactive scaffolds. Curr. Org. Chem. 2018, 22, 818−830. https://doi.org/10.2174/1385272822666180122153839
Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Calculation of the Global and Local Conceptual DFT Indices for the Prediction of the Chemical Reactivity Properties of Papuamides A-F Marine Drugs. Molecules. 2019, 24 (18), 3312. https://doi.org/10.3390/molecules24183312
Forgacs, P.; Wengenack, N. L.; Hall, L.; Zimmerman, S. K.; Silverman, M. L.; Roberts, G. D. Tuberculosis and Trimethoprim-Sulfamethoxazole. Antimicrobial Agents Chemotherapy. 2009, 53, 4789–4793. https://doi.org/10.1128/AAC.01658-08
Hu, L.; Li, Z.-R.; Jiang, J.-D.; Boykin, D. W. Novel diaryl or heterocyclic sulfonamides as antimitotic agents. Anti-Cancer Agents Med. Chem. 2008, 8 (7), 739−745. https://doi.org/10.2174/187152008785914806
Krátký, M.; Vinsová, J.; Volková, M.; Buchta, V.; Trejtnar, F.; Stolaríková, J. Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. European Journal of Medicinal Chemistry. 2012, 50, 433–440. https://doi.org/10.1016/j.ejmech.2012.01.060
Lee, C., Yang, W., and Parr, R. G., Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785-789
Lipinski, C. A. Lead-and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies. 2004, 1 (4), 337–341. https://doi.org/10.1016/j.ddtec.2004.11.007
Manzanilla, B.; Robles, J. Conceptual DFT Reactivity Descriptors Computational Study of Graphene and Derivatives Flakes: Doped Graphene, Graphane, Fluorographene, Graphene Oxide, Graphyne, and Graphdiyne. Journal of Mexican Chemical Society. 2020, 64 (3), 238–252. https://doi.org/10.29356/jmcs.v64i3.1167
Masters, P. A.; O’Bryan, T. A.; Zurlo, J.; Miller, D. Q.; Joshi, N. Trimethoprim-Sulfamethoxazole Revisited. Archives of Internal Medicine. 2003, 163, 402–410. https://doi.org/10.1001/archinte.163.4.402
Oyebamiji, A. K.; Fadare, O. A.; Akintelu, S. A.; Semire, B. Biological Studies on Anthra[1,9‑cd]pyrazol‑6(2D)‑one Analogues as Anti‑vascular Endothelial Growth Factor Via In silico Mechanisms. Chemistry Africa. 2021, 4, 955–963 https://doi.org/10.1007/s42250-021-00276-2
Oyebamiji, A. K.; Semire B. Dft-Qsar Model and Docking Studies of Antiliver Cancer (Hepg-2) Activities Of 1, 4-Diydropyridine Based Derivatives. Cancer Biology. 2016, 6 (2), 69–72. https://doi.org/10.7537/marscbj06021610
Oyeneyin, O. E. DFT and Monte Carlo simulations on the corrosion inhibitive potentials of some furan-based carbohydrazide derivatives. Letter of Applied NanoBio Science. 2023, 12 (4), 1–22. https://doi.org/10.33263/LIANBS124.113
Parr, R. G.; Szentpaly, L.; Liu, S. Electrophilicity index. Journal of American Chemical Society. 1999, 121 (9), 1922–1924. https://doi.org/10.1021/ja983494x
Ramírez-Martínez, C.; Zárate-Hernández, L. A.; Camacho-Mendoza, R. L.; González-Montiel, S.; Meneses-Viveros, A.; Cruz-Borbolla, J. The use of global and local reactivity descriptors of conceptual DFT to describe toxicity of benzoic acid derivatives. Computational and Theoretical Chemistry. 2023, 1226, 114211. https://doi.org/10.1016/j.comptc.2023.114211
Rasigade, J. P.; Vandenesch, F. Staphylococcus aureus: a pathogen with still unresolved issues. Infect. Genet. Evol. 2014, 21, 510–514. https://doi.org/10.1016/j.meegid.2013.08.018
Ratchanok, P.; Prasit, M.; Veda, P.; Anusit, T.; Supaluk, P.; Somsak, R.; Virapong, P. Investigations on Anticancer and Antimalarial Activities of Indole Sulfonamide Derivatives and In Silico Studies. ACS Omega. 2021, 6, 31854−31868. https://doi.org/10.1021/acsomega.1c04552
Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharmaceutica Sinica B. 2022, 12 (7), 3049–3062. https://doi.org/10.1016/j.apsb.2022.02.002
Supuran, C. T. Special Issue: Sulfonamides. Molecules. 2017, 22 (10), 1642. https://doi.org/10.3390/molecules22101642
Thanikaivelan, P., Subramanian, V.; Rao, J. R.; Nair, B. U. Application of quantum chemical descriptor in quantitative structure activity and structure property relationship. Chemical Physics Letter. 2000, 323 (1-2), 59–70. https://doi.org/10.1016/S0009-2614(00)00488-7
Trott, O.; Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. Journal of Computational Chemistry. 2010, 31, 455–461. https://doi.org/10.1002/jcc.21334
Hehre, W. J.; Radom, L.; Schleyer, P. V. R.; Pope, J. A. Ab initio molecular Orbital Theory, Wiley, New York, 1988
Waring, M. J.; Arrowsmith, J.; Leach, A. R.; Leeson, P. D.; Mandrell, S.; Owen, R. M. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug. Discov. 2015, 14, 475–486. https://doi.org/10.1038/nrd4609
Wertheim, H. F.; Melles, D. C.; Vos, M. C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H. A.; Nouwen, J. L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005, 5 (12), 751–762. https://doi.org/10.1016/S1473-3099(05)70295-4
Winum, J. Y.; Scozzafava, A.; Montero, J. L.; Supuran, C. T. Therapeutic potential of sulfamides as enzyme inhibitors. Med. Res. Rev. 2006, 26 (6), 767–792. https://doi.org/10.1002/med.20068
Yang, L.; Feng, J.; Ren, A. Theoretical studies on the electronic and optical properties of two thiophene–fluorene based π-conjugated copolymers. Polymer. 2005, 46 (24), 10970–10982. https://doi.org/10.1016/j.polymer.2005.09.050
Zhao, C.; Rakesh, K. P.; Ravidar, L.; Fang, W. Y.; Qin, H. L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679−734. https://doi.org/10.1016/j.ejmech.2018.11.017

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2025 Eclética Química