Abstract
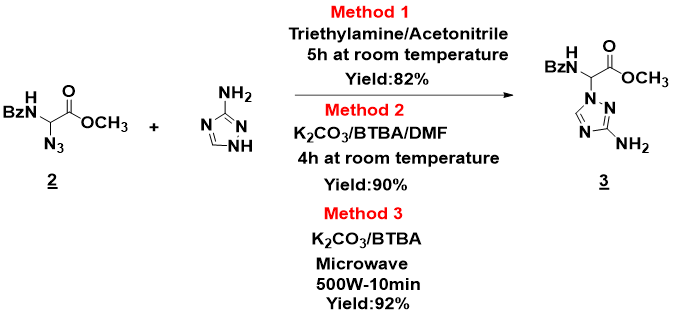
The interest of amino acids no longer needs to be demonstrated, given the involvement of these compounds in various fields, both as basic elements of peptide and protein structures and as independent entities. We report the regioselective synthesis of an N-protected α,α-diamino carboxylic ester derived from glycine. Our synthetic strategy is based first on the preparation of methyl 2-azido-2-benzamidoacetate and then on the N-alkylation reaction between the latter and the 1H-1,2,4-triazole-3-amine with three methods. The theoretical study by the DFT method and Marvinsketch software explains well the reaction's regioselectivity and good compatibility between the experimental and computational results. The products synthesized during this strategy are identified and characterized by spectral analysis: mass spectrometry, 1H NMR and 13C NMR.
References
Abboud, J.-L. M.; Foces-Foces, C.; Notario, R.; Trifonov, R. E.; Volovodenko, A. P.; Ostrovskii, V. A.; Alkorta, I.; Elguero, J. Basicity of N-H- and N-Methyl-1,2,3-Triazoles in the Gas Phase, in Solution, and in the Solid State − An Experimental and Theoretical Study. Eur. J. Org. Chem. 2001, 2001 (16), 3013–3024. https://doi.org/10.1002/1099-0690(200108)2001:16<3013::AID-EJOC3013>3.0.CO;2-Y
Abdu, T.; Adnan, A. B.; Yimer, S. Screening of some pyrazole derivatives as promising antileishmanial agent. African Journal of Pharmacy and Pharmacology. 2017, 11 (2), 32–37. https://doi.org/10.5897/AJPP2016.4401
Abu-Hashem, A. A.; Gouda, M. A. Synthesis and Antimicrobial Activity of Some Novel Quinoline, Chromene, Pyrazole Derivatives Bearing Triazolopyrimidine Moiety. Journal of Heterocyclic Chemistry. 2016, 54 (2), 850–858. https://doi.org/10.1002/jhet.2645
Achamlal, S.; Elachgar, A.; El Hallaoui, A.; El Hajji, S.; Roumestant, M. L.; Viallefont, Ph. Synthesis of α-Triazolyl α -Amino Acid Derivatives. Amino Acids.1997, 12 (3–4), 257–263. https://doi.org/10.1007/BF01373006
Albada, B.; Metzler-Nolte, N. Highly Potent Antibacterial Organometallic Peptide Conjugates. Acc. Chem. Res. 2017, 50 (10), 2510–2518. https://doi.org/10.1021/acs.accounts.7b00282
Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Review: biologically active pyrazole derivatives. New Journal of Chemistry. 2017, 41 (1), 16–41. https://doi.org/10.1039/C6NJ03181A
Azimi, F.; Azizian, H.; Najafi, M.; Hassanzadeh, F.; Sadeghi-aliabadi, H.; Ghasemi, J. B.; Ali Faramarzi, M.; Mojtabavi, S.; Larijani, B.; Saghaei, L.; Mahdavi, M. Design and Synthesis of Novel Quinazolinone-Pyrazole Derivatives as Potential α-Glucosidase Inhibitors: Structure-Activity Relationship, Molecular Modeling and Kinetic Study. Bioorganic Chem. 2021, 114, 105127. https://doi.org/10.1016/j.bioorg.2021.105127
Bandgar, B. P.; Gawande, S. S.; Bodade, R. G.; Gawande, N. M.; Khobragade, C. N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009, 17 (24), 8168–8173. https://doi.org/10.1016/j.bmc.2009.10.035
Banno, Y.; Sasaki, S.; Kamata, M.; Kunitomo, J.; Miyamoto, Y.; Abe, H.; Taya, N.; Oi, S.; Watanabe, M.; Urushibara, T.; Hazama, M.; Niwa, S.; Miyamoto, S.; Horinouchi, A.; Kuroshima, K.; Amano, N.; Matsumoto, S.; S.; Matsunaga, S. Design and synthesis of a novel series of orally active, selective somatostatin receptor 2 agonists for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2017, 25, 5995–6006. https://doi.org/10.1016/j.bmc.2017.09.031
Boibessot, T.; Bnimlis, D.; Jean, M.; Benfodda, Z.; Meffre, P. Synthesis of a Novel Rhizobitoxine-Like Triazole-Containing Amino Acid. Synlett. 2016, 27, 2685–2688. https://doi.org/10.1055/s-0036-1588300
Cativiela, C.; Díaz-de-Villegas, M. D. Recent Progress on the Stereoselective Synthesis of Acyclic Quaternary α-Amino Acids. Tetrahedron Asymmetry. 2007, 18 (5), 569–623. https://doi.org/10.1016/j.tetasy.2007.02.003.
Cetin, A.; Bildirici, I. A study on the synthesis and antimicrobial activity of 4-acyl-pyrazoles. J. Saudi Chem. Soc. 2018, 22 (3) 279–296. https://doi.org/10.1016/j.jscs.2016.05.008
Christian, O. E.; Compton, J.; Christian, K. R.; Mooberry, S. L.; Valeriote, F. A.; Crews, P. Using Jasplakinolide to Turn on Pathways That Enable the Isolation of New Chaetoglobosins from Phomospis Asparagi. J. Nat. Prod. 2005, 68 (11), 1592–1597. https://doi.org/10.1021/np050293f
Pelliciari, R.; Raimondo, M.; Marinozzi, M.; Natalini, B.; Costantino, G.; Thomsen, C. (S)-(+)-2-(3‘-Carboxybicyclo[1.1.1]pentyl)-glycine, a Structurally New Group I Metabotropic Glutamate Receptor Antagonist. Journal of Medicinal Chemistry. 1996, 39, 2874-2876. https://doi.org/10.1021/jm960254o
Diculescu, V. C.; Chiorcea-Paquim, A.-M.; Oliveira-Brett, A. M. Applications of a DNA-Electrochemical Biosensor. Trends Anal. Chem. 2016, 79, 23–36. https://doi.org/10.1016/j.trac.2016.01.019
Dondoni, A.; Massi, A. Design and Synthesis of New Classes of Heterocyclic C-Glycoconjugatesand Carbon-Linked Sugar and Heterocyclic Amino Acids by Asymmetric Multicomponent Reactions (AMCRs). Acc. Chem. Res. 2006, 39, 451–463. https://doi.org/10.1021/ar068023r
El-Sabbagh, O. I.; Baraka, M. M.; Ibrahim, S. M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Rashad, A. A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009, 44 (9), 3746–3753. https://doi.org/10.1016/j.ejmech.2009.03.038
Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F. A. Recent Developments in Synthetic Chemistry and Biological Activities of Pyrazole Derivatives. J. Chem. Sci. 2019, 131, 70. https://doi.org/10.1007/s12039-019-1646-1
Graillot, V.; Tomasetig, F.; Cravedi, J.-P.; Audebert, M. Evidence of the in Vitro Genotoxicity of Methyl-Pyrazole Pesticides in Human Cells. Mutat. Res. 2012, 748 (1–2), 8–16. https://doi.org/10.1016/j.mrgentox.2012.05.014
Hughes, R. A.; Moody, C. J. From Amino Acids to Heteroaromatics—Thiopeptide Antibiotics, Nature's Heterocyclic Peptides. Angew. Chem. Int. Ed. 2007a, 46 (42), 7930–7954. https://doi.org/10.1002/anie.200700728
Hughes, R. A.; Moody, C. J. Von Aminosäuren zu Heteroarenen – Thiopeptid-Antibiotika als heterocyclische Peptide aus der Natur. Angew. Chem. 2007b, 119 (42), 8076–8101. https://doi.org/10.1002/ange.200700728
Insuasty, B.; Tigreros, A.; Orozco, F.; Quiroga, J.; Abonia, R.; Nogueras, M.; Sanches, A.; Cobo, J. Synthesis of novel pyrazolic analogs of chalcones and their 3-aryl-4-(3-aryl-4, 5- dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18 (14), 4965–4974. https://doi.org/10.1016/j.bmc.2010.06.013
Jorgensen, C. G.; Bräuner-Osborne, H.; Nielsen, B.; Kehler, J.; Clausen, R. P.; Krogsgaard-Larsena, P.; Madsena, U. Novel 5-substituted 1-pyrazolol analogues of ibotenic acid: Synthesis and pharmacology at glutamate receptors.Bioorg. Med. Chem. 2007, 15 (10), 3524-3538. https://doi.org/10.1016/j.bmc.2007.02.047
Junaid, A.; Lim, P. L. F.; Zhou, Y. P.; Chui, W. K.; Dolzhenko. A. V. Fused Heterocyclic Systems with an s-Triazine Ring. 34. Development of a Practical Approach for the Synthesis of 5-Aza-iso guanines. Molecules. 2019, 24, 1453. https://doi.org/10.3390/molecules24081453
Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.; Al-aizari F.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules. 2018, 23 (1), 134. https://doi.org/10.3390/molecules23010134
Khilkovets, A.; Karpenko, Y.; Bigdan, O.; Parchenko, M.; Parchenko, V. Synthetic and biological aspects of studying the properties of 1,2,4-triazole derivatives. Scientific Journal of Polonia University. 2022, 51 (2), 324–331. https://doi.org/10.23856/5138
Knorr, L. Einwirkung von Acetessigester Auf Phenylhydrazin. Berichte Dtsch. Chem. Ges. 1883, 16 (2), 2597–2599. https://doi.org/10.1002/cber.188301602194
Kumar, V.; Kaur, K.; Gupta, G. K.; Sharma, A. K. Pyrazole Containing Natural Products: Synthetic Preview and Biological Significance. Eur. J. Med. Chem. 2013, 69, 735–753. https://doi.org/10.1016/j.ejmech.2013.08.053
Mabrouk, E. H.; Elachqar, A.; El Hallaoui, A.; Alami, A. Synthesis of New Racemic α,α-Diaminocarboxylic Ester Derivatives. Molecules. 2010, 13, 9354-9363. https://doi.org/10.3390/molecules15129354
Mabrouk, E. H.; Elachqar, A.; El Hallaoui, A.; Alami, A.; El Hajji, S.; Martinez, J.; Rolland, V. Synthesis of new racemic α-heterocyclic α, α-diamino esters and α-aminoester carboxylic. Arabian Journal of Chemistry. 2013, 6, 93–96. https://doi.org/10.1016/j.arabjc.2010.09.023
Mabrouk, E. H.; Arrousse, N.; Korchi, A.; Lachgar, M.; Oubair, A.; Elachqar, A.; Jabha, M.; Lachkar, M.; El hajjaji, F.; Rais, Z.; Taleb, M. Intelligence Way from Eco-friendly Synthesis Strategy of New Heterocyclic Pyrazolic Carboxylic α-Amino Esters. Chem. Res. Chinese Universities. 2020, 6, 1–7. https://doi.org/10.1007/s40242-020-0173-4
Nájera, C.; Sansano, J. M. Catalytic Asymmetric Synthesis of Alpha-Amino Acids. Chem. Rev. 2007, 107 (11), 4584–4671. https://doi.org/10.1021/cr050580o
Ngo, J. T.; Tirrell, D. A. Noncanonical Amino Acids in the Interrogation of Cellular Protein Synthesis. Acc. Chem. Res. 2011, 44 (9), 677–685. https://doi.org/10.1021/ar200144y
Ouyang, G.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food. Chem. 2008, 56 (21) 10160–10167. https://doi.org/10.1016/j.bmc.2008.09.070
Ozdemir, A.; Altıntop, M.D.; Kaplancıklı, Z.A.; Can, O.D.; Demir Ozkay, U.; Turan- Zitouni, G. Synthesis and evaluation of new 1, 5-diaryl-3-[4-(methyl-sulfonyl) phenyl]- 4, 5-dihydro-1h-pyrazole derivatives as potential antidepressant agents. Molecules. 2015, 20 (2), 2668–2684. https://doi.org/10.1002/ardp.201200479
Perez-Fernandez, R.; Goya, P.; Elguero, J. A review of recent progress (2002–2012) on the biological activities of pyrazoles, Arkivoc: Onl. J. Org. Chem. 2014, 2013, 233–293. https://doi.org/10.3998/ark.5550190.p008.131
Radi, S.; El Massaoudi, M.; Bacquet, M.; Degoutin, S.; Adarsh, N.N.; Robeyns, K.; Garcia, Y. A novel environment-friendly hybrid material based on a modified silica gel with a bis pyrazole derivative for the removal of Zn II, Pb II, Cd II, and Cu II traces from aqueous solutions. Inorg. Chem. Front. 2017, 4 (11) 1821–1831. https://doi.org/10.1039/C7QI00322F
Ramesh, B.; Bhalgat, C. M. Novel dihydropyrimidines, and its pyrazole derivatives: synthesis and pharmacological screening. Eur. J. Med. Chem. 2011, 46 (5), 1882–1891. https://doi.org/10.1016/j.ejmech.2011.02.052
Ramirez-Prada, J.; Robledo, S. M.; Velez, I. D.; del Pilar Crespo, M.; Quiroga, J.; Abonia, R.; Insuasty, B. Synthesis of novel quinoline-based 4,5-dihydro-1H-pyrazoles as potential anticancer, antifungal, antibacterial and antiprotozoal agents. Eur. J. Med. Chem. 2017, 131, 237–254. https://doi.org/10.1016/j.ejmech.2017.03.016
Rhazi, Y.; Chalkha, M.; Nakkabi, A.; Hammoudan, I.; Akhazzane, M.; Bakhouch, M.; Chtita, S.; El Yazidi, M. Novel Quinazolinone–Isoxazoline Hybrids: Synthesis, Spectroscopic Characterization, and DFT Mechanistic Study. Chemistry. 2022, 4, 969–982. https://doi.org/10.3390/chemistry4030066
Risseeuw, M.; Overhand, M.; Fleet, G. W. J.; Simone, M. I. A Compendium of Cyclic Sugar Amino Acids and Their Carbocyclic and Heterocyclic Nitrogen Analogues. Amino Acids. 2013, 45 (4), 613–689. https://doi.org/10.1007/s00726-013-1521-1
Schenk, S. U.; Werner, D. β-(3-isoxazolin-5-on-2-yl)-alanine from Pisum: Allelopathic properties and antimycotic bioassay - ScienceDirect. Photochemistry. 1991, 30, 467–470. https://doi.org/10.1016/0031-9422(91)83706-Q
Shilpy, A.; Deepika, P.; Dhirender, K.; Girish, K. G.; Ajay, K. Combinatorial Chemistry & HighThroughput Screening. 2018, 21,194–203.
Stanley, N. J.; Hutchinson, M. R.; Kvist, T.; Nielsen, B.; Mathiesen, J. M.; Bräuner-Osborne, H.; Avery, T. D.; Tiekink, E. R. T.; Pedersen, D. S.; Irvine, R. J.; Abell, A. D.; Taylor, D. K. A New Metabotropic Glutamate Receptor Agonist with in Vivo Anti-Allodynic Activity. Bioorg. Med. Chem. 2010, 18 (16), 6089–6098. https://doi.org/10.1016/j.bmc.2010.06.051
Steglich, W.; Kober, R. Untersuchungen zur Reaktion von Acylaminobrommalonestern und Acylaminobromessigestern mit Trialkylphosphiten-eine einfache Synthese von 2-Amino-2-(diethoxyphosphoryl)Essigsäure Ethylester. Liebigs Ann Chem. 1983, 4, 599–609. https://doi.org/10.1002/jlac.198319830409
Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical-Biology Applications. Chem. Rev. 2013, 113 (7), 4905–4979. https://doi.org/10.1021/cr200409f
Vogt, H.; Brse, S. Recent approaches towards the asymmetric synthesis of α,α-disubstituted α-amino acids. Org. Biomol. Chem. 2007, 5, 406–430. https://doi.org/10.1039/B611091F
Zarrouk, A.; Zarrok, H.; Salghi, R.; Bouroumane, N.; Hammouti, B.; Al-Deyab, S. S.; Ebn Touhami, M.; Bouachrine, M.; Oudda, H.; Boukhris, S. The Adsorption and Corrosion Inhibition of 2-[Bis-(3,5-dimethyl-pyrazol-1-ylmethyl)- amino]-pentanedioic Acid on Carbon Steel Corrosion in 1.0 m HCl. Int J Electrochem. Sci. 2012, 8 (9), 10215–10232. https://doi.org/10.1016/S1452-3981(23)13198-1

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2024 Eclética Química